Abstract
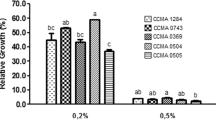
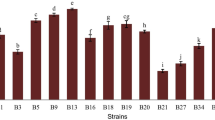
Three halotolerant lactobacilli (Lactobacillus plantarum, L. pentosus, and L. acidipiscis) isolated from a ripened Mexican tropical cheese (double cream Chiapas cheese) were evaluated as potential probiotics and compared with two commercial probiotic strains (L. casei Shirota and L. plantarum 299v) from human origin. All the strains survived the in vitro gastrointestinal simulation from the oral cavity to the ileum. During the stomach simulation, all the strains survived in satiety conditions (60 min, pH 3.0, 3 g/L pepsin, 150 rpm) and only L. pentosus could not survive under fasting conditions (60 min, pH 2.0, 3 g/L pepsin, 150 rpm). All the strains showed a strong hydrophilic character with low n-hexadecane and a variable chloroform affinity. L. plantarum showed a mucin adhesion rate similar to that of L. plantarum 299v and L. casei Shirota, while L. pentosus and L. acidipiscis had a lower mucin adhesion. The isolated halotolerant lactobacilli exhibited similar antimicrobial activity against some gram-positive and gram-negative pathogens in comparison with the two commercial strains. In addition, the proteinaceous character of the antimicrobial agents against the most pathogenic strains was demonstrated. The compounds showed a low molecular weight (less than 10 kDa). Besides, L. plantarum and L. acidipiscis were able to produce the enzyme β-galactosidase. Finally, L. pentosus was able to deconjugate taurocholic, taurodeoxycholic, glycocholic, and glycodeoxycholic acids better than the two commercial strains analyzed. All these results suggest that the halotolerant lactobacilli isolated from this ripened Mexican cheese could be potentially probiotic. This is the first time that halotolerant lactic acid bacteria have been shown to have probiotic properties.


Similar content being viewed by others
References
Ambalam PS, Prajapati JB, Dave JM, Nair BM, Ljungh Å, Vyas BRM (2009) Isolation and characterization of antimicrobial proteins produced by a potential probiotic strain of human Lactobacillus rhamnosus 231 and its effect on selected human pathogens and food spoilage organisms. Microb Ecol Health Dis 21(3–4):211–220
Amerongen AVN, Veerman ECI (2002) Saliva—the defender of the oral cavity. Oral Dis 8:12–22
Balciunas EM, Castillo Martinez FA, Todorov SD, Gombossy de Melo Franco BD, Converti A, Pinheiro de Souza Oliveira R (2012) Novel biotechnological applications of bacteriocins: a review. Food Control 32:134–142
Begley M, Hill C, Gahan CGM (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72:1729–1738
Bellon-Fontaine MN, Rault J, Van Oss CJ (1996) Microbial adhesion to solvents: a novel method to determine the electron-donor/electron-acceptor or Lewis acid–base properties of microbial cells. Colloids Surf B 7(1):47–53
Bucio A, Hartemink R, Schrama JW, Verreth J, Rombouts FM (2005) Survival of Lactobacillus plantarum 44a after spraying and drying in feed and during exposure to gastrointestinal tract fluids in vitro. J Gen Appl Microbiol 51:221–227
Chen J, Gaikwad V, Holmes M, Murray B, Povey M, Wang Y, Zhang Y (2011) Development of a simple model device for in vitro gastric digestion investigation. Food Funct 2:174–182
Collado M, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226:1065–1073
Collado MC, Gueimonde M, Salminen S (2010) Probiotics in adhesion of pathogens: mechanisms of action. In: Watson RR, Preedy VR (eds) Bioactive foods in promoting health. Probiotics and probiotics. Academic Press, Boston, pp 353–370
Colloca ME, Ahumada MC, López ME, Nader-Macías ME (2000) Surface properties of lactobacilli isolated from healthy subjects. Oral Dis 6:227–233
Cotter PD, Ross RP, Hill C (2013) Bacteriocins a viable alternative to antibiotics? Nat Rev Microbiol 11(2):95–105
de Vries MC, Vaughan EE, Kleerebezem M, de Vos WM (2006) Lactobacillus plantarum—survival, functional and potential probiotic properties in the human intestinal tract. Int Dairy J 16:1018–1028
Deepika G, Charalampopoulos D (2010) Surface and adhesion properties of Lactobacilli. Adv Appl Microbiol 70:127–152
Doyle P, Meng J (2006) Bacteria in food and beverage production. In: Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes. Vol. 1. Symbiotic associations, biotechnology, applied microbiology, 3rd edn. Springer, New York, pp 797–811
FAO/WHO (2002) Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in foods. FAO/WHO, London
Gagnon M, Zihler A, Chassard C, Lacroix C (2011) Ecology of probiotics and enteric protection. In: Malago JJ, Koninkx JFJJ, Marinsek-Logar R (eds) Probiotic bacteria and enteric infections. Springer, Dordrecht, pp 65–85
Gbassi GK, Vandamme T (2012) Probiotic encapsulation technology: from microencapsulation to release into the gut. Pharmaceutics 4:149–163
González-Sánchez F, Azaola A, Gutiérrez-López GF, Hernández-Sánchez H (2010) Viability of microencapsulated Bifidobacterium animalis ssp. lactis BB12 in kefir during refrigerated storage. Int J Dairy Technol 63:431–436
Guinee TP, Fox PF (2004) Salt in cheese: physical, chemical and biological aspects. Cheese Chem Phys Microbiol 1:207–259
Ivanova I, Miteva V, Stefanova T, Pantev A, Budakov I, Danova S, Moncheva P, Nikolova I, Dousset X, Boyaval P (1998) Characterization of a bacteriocin produced by Streptococcus thermophilus 81. Int J Food Microbiol 42(3):147–158
Iyer R, Tomar S, Kapila S, Mani J, Singh R (2010) Probiotic properties of folate producing Streptococcus thermophilus strains. Food Res Int 43:103–110
Kos B, Šušković J, Vuković S, Šimpraga M, Frece J, Matošić S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987
Koseki S, Mizuno Y, Sotome I (2011) Modeling of pathogen survival during simulated gastric digestion. Appl Environ Microbiol 77:1021–1032
Kumar R, Grover S, Batish VK (2012) Bile salt hydrolase (Bsh) activity screening of lactobacilli: in vitro selection of indigenous lactobacillus strains with potential bile salt hydrolysing and cholesterol-lowering ability. Probiotics Antimicrob Proteins 4:162–172
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lash BW, Gourama H, Mysliwiec TH (2002) Microscale assay for screening of inhibitory activity of Lactobacillus. Biotechniques 33:1224–1228
Liong M, Shah N (2005) Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. Int Dairy J 15:391–398
Ljungh A, Wadström T (2006) Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7:73–90
Lo Curto A, Pitino I, Mandalari G, Dainty JR, Faulks RM, John Wickham MS (2011) Survival of probiotic lactobacilli in the upper gastrointestinal tract using an in vitro gastric model of digestion. Food Microbiol 28:1359–1366
Madureira AR, Amorim M, Gomes AM, Pintado ME, Malcata FX (2011) Protective effect of whey cheese matrix on probiotic strains exposed to simulated gastrointestinal conditions. Food Res Int 44:465–470
Mathara JM, Schillinger U, Guigas C, Franz C, Kutima PM, Mbugua SK, Shin HK, Holzapfel WH (2008) Functional characteristics of Lactobacillus spp. from traditional Maasai fermented milk products in Kenya. Int J Food Microbiol 126:57–64
Morales F, Morales J, Hernández C, Hernández-Sánchez H (2011) Isolation and partial characterization of halotolerant lactic acid bacteria from two Mexican cheeses. Appl Biochem Biotechnol 164:889–905
Nguyen TDT, Kang JH, Lee MS (2007) Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol 113:358–361
Oelschlaeger TA (2010) Mechanisms of probiotic actions—a review. Int J Med Microbiol 300:57–62
Ofek I, Hasty DL, Sharon N (2003) Anti-adhesion therapy of bacterial diseases: prospects and problems. FEMS Immunol Med Microbiol 38:181–191
Pacheco KC, Valencia-Del Toro G, Martinez FR, Durán-Páramo E (2010) Viability of Lactobacillus delbrueckii under human gastrointestinal conditions simulated in vitro. Am J Agric Biol Sci 5:37–42
Parada JL, Caron CR, Medeiros ABP, Soccol CR (2007) Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Brazilian Arch Biol Technol 50(3):512–542
Patel AK, Singhania RR, Pandey A, Chincholkar SB (2010) Probiotic bile salt hydrolase: current developments and perspectives. Appl Biochem Biotechnol 162:166–180
Reis JA, Paula AT, Casarotti SN, Penna ALB (2012) Lactic acid bacteria antimicrobial compounds: characteristics and applications. Food Eng Rev 4(2):124–140
Ridlon JM, Kang DJ, Hylemon PB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Sako T (2010) The world’s oldest probiotic: perspectives for health claims. In: Kneifel W, Salminen S (eds) Probiotics and health claims. Wiley-Blackwell, Oxford, pp 17–36
Salminen SJ (1998) Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol 41:45–51
Sánchez B, Bressollier P, Urdaci MC (2008) Exported proteins in probiotic bacteria: adhesion to intestinal surfaces, host immunomodulation and molecular cross-talking with the host. FEMS Immunol Med Microbiol 54:1–17
Sutula J, Coulthwaite L, Verran J (2012) Culture media for differential isolation of Lactobacillus casei Shirota from oral samples. J Microbiol Methods 90:65–71
Tallon R, Arias S, Bressollier P, Urdaci MC (2007) Strain- and matrix-dependent adhesion of Lactobacillus plantarum is mediated by proteinaceous bacterial compounds. J Appl Microbiol 102:442–451
Vamanu E, Vamanu A, Nita S, Rusu N (2011) The viability of the Lactobacillus paracasei IL2 and Lactobacillus plantarum IL3 strains in simulated gastrointestinal conditions. Afr J Microbiol Res 5:1029–1036
Van den Abbeele P, Grootaert C, Possemiers S, Verstraete W, Verbeken K, Van de Wiele T (2009) In vitro model to study the modulation of the mucin-adhered bacterial community. Appl Microbiol Biotechnol 83:349–359
Vinderola C, Reinheimer J (2003) Lactic acid starter and probiotic bacteria: a comparative. Food Res Int 36:895–904
Vinderola G, Capellini B, Villarreal F, Suárez V, Quiberoni A, Reinheimer J (2008) Usefulness of a set of simple in vitro tests for the screening and identification of probiotic candidate strains for dairy use. LWT-Food Sci Technol 41:1678–1688
Walter J (2008) Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol 74(16):4985–4996
Zago M, Fornasari ME, Carminati D, Burns P, Suárez V, Vinderola G, Reinheimer J, Giraffa G (2011) Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol 28:1033–1040
Acknowledgments
This research was supported by Consejo Nacional de Ciencia y Tecnología (Conacyt) through the grant number 206847 and Instituto Politécnico Nacional with the project number SIP-20110353.
Conflict of interest
Neither author has any conflict of interest in performing and reporting these experiments. This includes the fact that neither author has any link, direct or indirect, to any of the manufacturers of the commercial strains used.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melgar-Lalanne, G., Rivera-Espinoza, Y., Reyes Méndez, A.I. et al. In Vitro Evaluation of the Probiotic Potential of Halotolerant Lactobacilli Isolated from a Ripened Tropical Mexican Cheese. Probiotics & Antimicro. Prot. 5, 239–251 (2013). https://doi.org/10.1007/s12602-013-9144-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-013-9144-0