Abstract
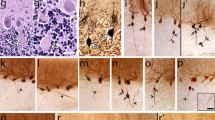
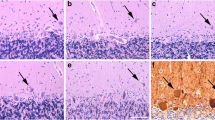
Torpedo formation and Purkinje cell (PC) loss represent standard and inter-related cerebellar responses to injury. Surprisingly, the nature of their relationship has not been carefully characterized across a range of normal and disease states. Are brains with more torpedoes expected to have fewer PCs? We quantified torpedoes and PCs in four groups: essential tremor (ET), spinocerebellar ataxia (SCA), multiple system atrophy-cerebellar (MSA-C), and controls. Brains from 100 individuals (58 ET, 27 controls, 7 SCA, 8 MSA-C) were available at the New York Brain Bank. After complete neuropathological assessment, a standard parasagittal neocerebellar block was harvested; a 7-μm thick section was stained with Luxol fast blue/hematoxylin and eosin; and torpedoes and PCs were quantified. For a given PC count, SCA and MSA-C cases often had higher torpedo counts than ET cases or controls. Furthermore, the relationship between torpedo and PC counts was complex. The correlation between torpedo and PC counts was negative in ET cases (i.e., individuals with more torpedoes had fewer PCs [i.e., more PC loss]) whereas the relationship was positive in MSA-C cases (i.e., individuals with fewer PCs [i.e., more PC loss] had fewer torpedoes). Patients with SCA showed both patterns. When all diagnostic groups were combined, the correlation was best fit by a quadratic (i.e., parabolic) model rather than a simple linear model; this model incorporated data on the negative correlation in ET cases, the mixed results in SCA cases, and the positive correlation in MSA-C cases (r = 0.636). The relationship between torpedo and PC counts was complex and heterogeneous across a range of cerebellar disease states, and was best characterized by a quadratic rather than a simple model. With more severe cerebellar disease, torpedoes can be quite numerous and are likely a common feature of surviving PCs, but eventually, dramatic loss of PC leads to a paradoxical reduction in observable torpedoes.


Similar content being viewed by others
References
Mizushima S. An ultrastructural observation of torpedoes in the human degenerative cerebellum. J Clin Electron Microsc. 1976;9:672–3.
Mann DM, Stamp JE, Yates PO, Bannister CM. The fine structure of the axonal torpedo in Purkinje cells of the human cerebellum. Neurol Res. 1980;1:369–78.
Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307.
Louis ED, Faust PL, Vonsattel JP, et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord. 2009;24:1600–5.
Louis ED, Faust PL, Vonsattel JP, Erickson-Davis C. Purkinje cell axonal torpedoes are unrelated to advanced aging and likely reflect cerebellar injury. Acta Neuropathol. 2009;117:719–21.
Takahashi N, Iwatsubo T, Nakano I, Machinami R. Focal appearance of cerebellar torpedoes associated with discrete lesions in the cerebellar white matter. Acta Neuropathol. 1992;84(2):153–6.
Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–61.
Bares M, Lungu OV, Husarova I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–35.
Cerasa A, Messina D, Nicoletti G, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. Am J Neuroradiol. 2009;30(6):1240–3.
Benito-Leon J. Essential tremor: one of the most common neurodegenerative diseases? Neuroepidemiology. 2011;36:77–8.
Rossi F, Jankovski A, Sotelo C. Differential regenerative response of Purkinje cell and inferior olivary axons confronted with embryonic grafts: environmental cues versus intrinsic neuronal determinants. J Comp Neurol. 1995;359:663–77.
Bravin M, Savio T, Strata P, Rossi F. Olivocerebellar axon regeneration and target reinnervation following dissociated Schwann cell grafts in surgically injured cerebella of adult rats. Eur J Neurosci. 1997;9:2634–49.
Dusart I, Sotelo C. Lack of Purkinje cell loss in adult rat cerebellum following protracted axotomy: degenerative changes and regenerative attempts of the severed axons. J Comp Neurol. 1994;347:211–32.
Rossi F, Jankovski A, Sotelo C. Target neuron controls the integrity of afferent axon phenotype: a study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J Neurosci. 1995;15:2040–56.
Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–61.
Scherzed W, Brunt ER, Heinsen H, et al. Pathoanatomy of cerebellar degeneration in spinocerebellar ataxia type 2 (SCA2) and type 3 (SCA3). Cerebellum. 2012;1:749–60.
Auburger GW. Spinocerebellar ataxia type 2. Handb Clin Neurol. 2012;103:423–36.
Hellenbroich Y, Gierga K, Reusche E, et al. Spinocerebellar ataxia type 4 (SCA4): Initial pathoanatomical study reveals widespread cerebellar and brainstem degeneration. J Neural Transm. 2006;113:829–43.
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21.
Donato SD, Mariotti C, Taroni F. Spinocerebellar ataxia type 1. Handb Clin Neurol. 2012;103:399–421.
Hoche F, Baliko L, den Dunnen W, et al. Spinocerebellar ataxia type 2 (SCA2): identification of early brain degeneration in one monozygous twin in the initial disease stage. Cerebellum. 2011;10:245–53.
Solodkin A, Gomez CM. Spinocerebellar ataxia type 6. Handb Clin Neurol. 2012;103:461–73.
Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–4.
Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–7.
Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6.
Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–35.
Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–6.
Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–8.
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18:S91–4.
Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66:1756–9.
Miyamae M, Kaneda K, Domae N, Figueredo VM. Cardioprotection by regular ethanol consumption: potential mechanisms and clinical application. Curr Drug Abus Rev. 2010;3:39–48.
Wesley D, Cox HF. Modeling total cholesterol as predictor of mortality: the low-cholesterol paradox. J Insur Med. 2011;42:62–75.
Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9:613–22.
Erickson-Davis CRFP, Vonsattel JGV, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–71.
Yu M, Ma K, Faust PL, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19:625–30.
Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82:1038–40.
Louis ED, Yi H, Erickson-Davis C, Vonsattel JP, Faust PL. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett. 2009;450:287–91.
Beaulieu JM, Nguyen MD, Julien JP. Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol. 1999;147:531–44.
Liem RK, Leung CL. Neuronal intermediate filament overexpression and neurodegeneration in transgenic mice. Exp Neurol. 2003;184:3–8.
Acknowledgements
Tissue samples from six SCA-1 cerebellum were provided by Dr. Arnulf H. Koeppen, Albany, NY, on behalf of the National Ataxia Foundation. This work was funded by NIH R01NS042859.
Conflict of Interest
There are no conflicts of interest or competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Louis, E.D., Kuo, SH., Vonsattel, JP.G. et al. Torpedo Formation and Purkinje Cell Loss: Modeling their Relationship in Cerebellar Disease. Cerebellum 13, 433–439 (2014). https://doi.org/10.1007/s12311-014-0556-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-014-0556-5