Abstract
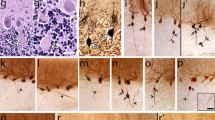
Essential tremor (ET) is among the most common neurological diseases. Postmortem studies have noted a series of pathological changes in the ET cerebellum. Heterotopic Purkinje cells (PCs) are those whose cell body is mis-localized in the molecular layer. In neurodegenerative settings, these are viewed as a marker of the progression of neuronal degeneration. We (1) quantify heterotopias in ET cases vs. controls, (2) compare ET cases to other cerebellar degenerative conditions (spinocerebellar ataxias (SCAs) 1, 2, 3, and 6), (3) compare these SCAs to one another, and (4) assess heterotopia within the context of associated PC loss in each disease. Heterotopic PCs were quantified using a standard LH&E-stained section of the neocerebellum. Counts were normalized to PC layer length (n-heterotopia count). It is also valuable to consider PC counts when assessing heterotopia, as loss of PCs extends both to normally located as well as heterotopic PCs. Therefore, we divided n-heterotopias by PC counts. There were 96 brains (43 ET, 31 SCA [12 SCA1, 7 SCA2, 7 SCA3, 5 SCA6], and 22 controls). The median number of n-heterotopias in ET cases was two times higher than that of the controls (2.6 vs. 1.2, p < 0.05). The median number of n-heterotopias in the various SCAs formed a spectrum, with counts being highest in SCA3 and SCA1. In analyses that factored in PC counts, ET had a median n-heterotopia/Purkinje cell count that was three times higher than the controls (0.35 vs. 0.13, p < 0.01), and SCA1 and SCA2 had counts that were 5.5 and 11 times higher than the controls (respective p < 0.001). The median n-heterotopia/PC count in ET was between that of the controls and the SCAs. Similarly, the median PC count in ET was between that of the controls and the SCAs; the one exception was SCA3, in which the PC population is well known to be preserved. Heterotopia is a disease-associated feature of ET. In comparison, several of the SCAs evidenced even more marked heterotopia, although a spectrum existed across the SCAs. The median n-heterotopia/PC count and median PC in ET was between that of the controls and the SCAs; hence, in this regard, ET could represent an intermediate state or a less advanced state of spinocerebellar atrophy.



Similar content being viewed by others
References
Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41.
Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province. Turkey Neurology. 2003;61:1804–6.
Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–94.
Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013;20:725–7.
Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. 2009;24:626–7.
Koster B, Deuschl G, Lauk M, Timmer J, Guschlbauer B, Lucking CH. Essential tremor and cerebellar dysfunction: abnormal ballistic movements. J Neurol Neurosurg Psychiatry. 2002;73:400–5.
Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9:193–6.
Hoskovcova M, Ulmanova O, Sprdlik O, Sieger T, Novakova J, Jech R, et al. Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum. 2012;12:27–34.
Louis ED, Galecki M, Rao AK. Four essential tremor cases with moderately impaired gait: how impaired can gait be in this disease? Tremor Other Hyperkinet Mov (N Y). 2013;3. pii: tre-03-200-4597-1. doi: 10.7916/D8QV3K7G. eCollection 2013.
Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001;124:2278–86.
Helmchen C, Hagenow A, Miesner J, Sprenger A, Rambold H, Wenzelburger R, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126:1319–32.
Gitchel GT, Wetzel PA, Baron MS. Slowed saccades and increased square wave jerks in essential tremor. Tremor Other Hyperkinet Mov (N Y). 2013;3. pii: tre-03-178-4116-2. doi: 10.7916/D8251GXN. eCollection 2013.
Bares M, Lungu OV, Husarova I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–35.
Benito-Leon J, Labiano-Fontcuberta A. Linking essential tremor to the cerebellum: clinical evidence. Cerebellum. 2016;15:253–62.
Cerasa A, Quattrone A. Linking essential tremor to the cerebellum-neuroimaging evidence. Cerebellum. 2016;15:263–75.
Passamonti L, Cerasa A, Quattrone A. Neuroimaging of essential tremor: what is the evidence for cerebellar involvement? Tremor Other Hyperkinet Mov (N Y). 2012;2. pii: 02–67–421-3. doi: 10.7916/D8F76B8G. Epub 2012 Sep 17.
Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333:17–20.
Sharifi S, Nederveen AJ, Booij J, van Rootselaar AF. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin. 2014;5:217–31.
Quattrone A, Cerasa A, Messina D, Nicoletti G, Hagberg GE, Lemieux L, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. Am J Neuroradiol. 2008;29:1692–7.
Louis ED. Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum. 2016;15:235–42.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307.
Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–61.
Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–71.
Kuo SH, Tang G, Louis ED, Ma K, Babji R, Balatbat M, et al. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 2013;125:879–89.
Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP. Quantification of cerebellar hemispheric Purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–9.
Choe M, Cortes E, Vonsattel JG, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord. 2016;31:393–401.
Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29:496–500.
Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–8.
Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82:1038–40.
Nakamura R, Kurita K, Kawanami T, Kato T. An immunohistochemical study of Purkinje cells in a case of hereditary cerebellar cortical atrophy. Acta Neuropathol. 1999;97:196–200.
Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, et al. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 1997;42:933–50.
Yamada M, Sato T, Tsuji S, Takahashi H. CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:71–86.
Mangaru Z, Salem E, Sherman M, Van Dine SE, Bhambri A, Brumberg JC, et al. Neuronal migration defect of the developing cerebellar vermis in substrains of C57BL/6 mice: cytoarchitecture and prevalence of molecular layer heterotopia. Dev Neurosci. 2013;35:28–39.
Bottini AR, Gatti RA, Wirenfeldt M, Vinters HV. Heterotopic Purkinje cells in ataxia-telangiectasia. Neuropathology. 2012;32:23–9.
Shahbazian MD, Orr HT, Zoghbi HY. Reduction of Purkinje cell pathology in SCA1 transgenic mice by p53 deletion. Neurobiol Dis. 2001;8:974–81.
Yang Q, Hashizume Y, Yoshida M, Wang Y, Goto Y, Mitsuma N, et al. Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathol. 2000;100:371–6.
Goffinet AM, So KF, Yamamoto M, Edwards M, Caviness VS Jr. Architectonic and hodological organization of the cerebellum in reeler mutant mice. Brain Res. 1984;318:263–76.
Kuo SH, Wang J, Tate WJ, Pan MK, Kelly GC, Gutierrez J, et al. Cerebellar pathology in early onset and late onset essential tremor. Cerebellum. 2017;16:473–82.
Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–35.
Louis ED, Babij R, Ma K, Cortes E, Vonsattel JP. Essential tremor followed by progressive supranuclear palsy: postmortem reports of 11 patients. J Neuropathol Exp Neurol. 2013;72:8–17.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501.
Koeppen AH, Ramirez RL, Bjork ST, Bauer P, Feustel PJ. The reciprocal cerebellar circuitry in human hereditary ataxia. Cerebellum. 2013;12:493–503.
Rub U, Schols L, Paulson H, Auburger G, Kermer P, Jen JC, et al. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol. 2013;104:38–66.
Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–59.
Acknowledgements
Nine control brains were from the Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA). Dr. Arnulf H. Koeppen, Veterans Affairs Medical Center, Albany, New York, USA, provided human SCA1, SCA2, and SCA6 tissues. Human SCA2 and SCA3 tissues were also obtained from the NIH NeuroBioBank at the University of Maryland, Baltimore, MD. An SCA3 human tissue specimen was also obtained from Dr. José Luiz Pedroso, Ataxia Unit Federal University of Sao Paulo, Sao Paulo, Brazil.
Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS046436 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator), and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University). Dr. Kuo has received funding from the National Institutes of Health: NINDS #K08 NS083738 (principal investigator), and the Louis V. Gerstner Jr. Scholar Award, Parkinson’s Disease Foundation, and International Essential Tremor Foundation. Dr. Vonsattel has received funding from the National Institutes of Health: NINDS #R01 NS088257 (coinvestigator) and NINDS #R01 NS046436 (coinvestigator). Dr. Faust has received funding from the National Institutes of Health: NINDS #R01 NS088257 (principal investigator) and NINDS #R01 NS085136 (principal investigator).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Louis, E.D., Kuo, SH., Tate, W.J. et al. Heterotopic Purkinje Cells: a Comparative Postmortem Study of Essential Tremor and Spinocerebellar Ataxias 1, 2, 3, and 6. Cerebellum 17, 104–110 (2018). https://doi.org/10.1007/s12311-017-0876-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0876-3