Abstract
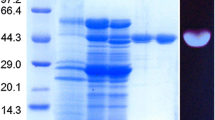
The effect of several carbon sources on the production of mycelial-bound β-glucosidase by Humicola grisea var. thermoidea in submerged fermentation was investigated. Maximum production occurred when cellulose was present in the culture medium, but higher specific activities were achieved with cellobiose or sugarcane bagasse. Xylose or glucose (1%) in the reaction medium stimulated β-glucosidase activity by about 2-fold in crude extracts from mycelia grown in sugarcane bagasse. The enzyme was purified by ammonium sulfate precipitation, followed by Sephadex G-200 and DEAE-cellulose chromatography, showing a single band in PAGE and SDS-PAGE. The β-glucosidase had a carbohydrate content of 43% and showed apparent molecular masses of 57 and 60 kDa, as estimated by SDS-PAGE and gel filtration, respectively. The optimal pH and temperature were 6.0 and 50°C, respectively. The purified enzyme was thermostable up to 60 min in water at 55°C and showed half-lives of 7 and 14 min when incubated in the absence or presence of 50 mM glucose, respectively, at 60°C. The enzyme hydrolyzed p-nitrophenyl-β-D-glucopyranoside, p-nitrophenyl-β-Dgalactopyranoside, p-nitrophenyl-β-D-fucopyranoside, p-nitrophenyl-β-D-xylopyranoside, o-nitrophenyl-β-Dgalactopyranoside, lactose, and cellobiose. The best synthetic and natural substrates were p-nitrophenyl-β-Dfucopyranoside and cellobiose, respectively. Purified enzyme activity was stimulated up to 2-fold by glucose or xylose at concentrations from 25 to 200 mM. The addition of purified or crude β-glucosidase to a reaction medium containing Trichoderma reesei cellulases increased the saccharification of sugarcane bagasse by about 50%. These findings suggest that H. grisea var. thermoidea β-glucosidase has a potential for biotechnological applications in the bioconversion of lignocellulosic materials.
Similar content being viewed by others
References
Andrade, S.V., M.L.T.M. Polizeli, H.F. Terenzi, and J.A. Jorge. 2004. Effect of carbon source on the biochemical properties of β-xylosidases produced by Aspergillus versicolor. Proc. Biochem. 39, 1931–1938.
Beguin, P. and J.P. Aubert. 1994. The biological degradation of cellulose. FEMS Microbiol. Rev. 13, 25–58.
Bergmeyer, H.U. and E. Bernt. 1974. D-glucose determination with glucose oxidase and peroxidase, pp. 1205–1215. In H.U. Bergmeyer (ed.), Methods of Enzymatic Analysis, vol. 3. Verlag Chimie-Academic Press, New York, N.Y., USA.
Bhat, M. and T.S. Bhat. 1997. Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 15, 583–620.
Bhatia, Y., S. Mishra, and V.S. Bisaria. 2002. Microbial β-glucosidases: cloning, properties, and applications. Crit. Rev. Biotechnol. 22, 375–407.
Bhiri, F., S.E. Chaabouni, F. Limam, R. Ghrir, and N. Marzouki. 2008. Purification and biochemical characterization of extracellular β-glucosidases from the hypercellulolytic Pol6 mutant of Penicillium occitanis. Appl. Biochem. Biotechnol. 149, 169–182.
Cooney, D.G. and R. Emerson. 1964. Humicola insolens and Humicola grisea var. thermoidea. Thermophilic fungi: an account of their biology, activities, and classification, pp. 73–79. In W.H. Freeman (ed.), San Francisco, California, USA.
Davis, B.J. 1964. Disc electrophoresis. II. Method and application to human serum proteins. Ann. N. Y. Acad. Sci. 121, 404–427.
Decker, C.H., J. Visser, and P. Schreier. 2001. β-glucosidase multiplicity from Aspergillus tubingiensis CBS 643.92: purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Appl. Microbiol. Biotechnol. 55, 157–163.
Dey, N.B., P. Bounelis, T.A. Fritz, D.M. Bedewell, and R.B. Marchase. 1994. The glycosylation of phosphoglumutase is modulated by carbon source and heat shock in Saccharomyces cerevisiae. J. Biol. Chem. 269, 27143–27148.
Dubois, M., K.A. Gilles, J.K. Hamilton, P.A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356.
Duff, S.J.B. and W.D. Murray. 1996. Bioconversion of forest products industry waste cellulosics to fuel ethanol: A review. Biores. Technol. 55, 1–33.
El-Hawary, F.I. and Y.S. Mostafa. 2001. Factors affecting cellulose production by Trichoderma koningii. Acta Aliment. 30, 3–13.
El-Hawary, F.I., Y.S. Mostafa, and E. Laszlo. 2001. Cellulase production and conversion of rice straw to lactic acid by simultaneous saccharification and fermentation. Acta Aliment. 30, 281–295.
Galbe, M. and G. Zacchi. 2002. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 59, 618–628.
Gao, J., H. Weng, D. Zhu, M. Yuan, F. Guan, and Y. Xi. 2008. Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation on corn stover. Biores. Technol. 99, 7623–7629.
Gusakov, A.V., T.N. Salanovich, A.I. Antonov, B.B. Ustinov, O.N. Okunev, R. Burlingame, M. Emalfarb, M. Baez, and A.P. Sinitsyn. 2007. Design of highly efficient cellulase mixtures for enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 97, 1028–1038.
Harchand, R.K. and S. Singh. 1997. Characterization of cellulose complex of Streptomyces albaduncus. J. Basic Microbiol. 37, 93–103.
Harrison, M.J., A.S. Nouwens, D.R. Jardine, N.E. Zachara, A.A. Gooley, and H. Nevalainen. 1998. Modified glycosylation of cellobiohydrolase I from a high cellulase producing mutant strain of Trichoderma reesei. Eur. J. Biochem. 256, 119–127.
Hayashida, S., K. Ohta, and K. Mo. 1988. Cellulases of Humicola insolens and Humicola grisea. Methods Enzymol. 160, 323–332.
Hui, J.P.M., P. Lanthier, T.C. White, S.G. McHugh, M. Yaguchi, R. Roy, and P. Tribault. 2001. Characterization of cellobiohydrolase I (Cel7A) glycoforms from extracts of Trichoderma reesei using capillary isoelectric focusing and electrospray mass spectrometry. J. Chrom. B 752, 349–368.
Karnchanatat, A., A. Petsom, P. Sangvanich, J. Piaphukiew, A.J. Whalley, C.D. Reynolds, and P. Sihanonth. 2007. Purification and biochemical characterization of an extracellular beta-glucosidase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.: Fr.) Rehm. FEMS Microbiol. Lett. 270, 162–170.
Kaur, J., B.S. Chadha, B.A. Kumar, S.K. Ghatora, and H.S. Saini. 2007. Purification and characterization of β-glucosidase from Melanocarpus sp. MTCC 3922. Electronic J. Biotechnol. 10, 260–270.
Kaur, J., B.S. Chadha, and H.S. Saini. 2006. Regulation of cellulose production in two thermophilic fungi Melanocarpus sp. MTCC 3922 and Scytalidium thermophilum MTCC 4520. Enzyme Microb. Technol. 38, 931–936.
Kern, G., N. Schülke, F.X. Schmid, and R. Jaenicke. 1992. Stability, quaternary structure, and folding of internal, external, and coreglycosylated invertase from yeast. Protein Sci. 1, 120–131.
Klarskov, K., K. Piens, J. Stahlberg, P.B. Hoj, J.M. Van Beeumen, and M. Claeyssens. 1997. Cellobiohydrolase I from Trichoderma reesei: Identification of an active-site nucelophile and additional information on sequence including the glycosylation pattern for the core protein. Carbohydr. Res. 304, 143–154.
Kumar, R., S. Singh, and O.V. Singh. 2008. Bioconversion of lingocellulosic biomass: biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 35, 377–391.
Leite, R.S.R., H.F. Alves-Prado, H. Cabral, F.C. Pagnocca, E. Gomes, and R. Da-Silva. 2008. Production and characteristics comparison of crude β-glucosidases produced by microorganisms Thermoascus aurantiacus e Aureobasidium pullulans in agricultural wastes. Enzyme Microb. Technol. 43, 391–395.
Leone, F.A., J.A. Baranauskas, R.P.M. Furriel, and I.A. Borin. 2005. SigrafW: an easy-to-use program for fitting enzyme kinetic data. Biochem. Mol. Biol. Educ. 33, 399–403.
Lige, B., S. Ma, and R.B. van Huystee. 2001. The effects of the sitedirected removal of N-glycosylation from cationic peanut peroxidase on its function. Arch. Biochem. Biophys. 386, 17–24.
Lin, J., B. Pillay, and S. Singh. 1999. Purification and biochemical characteristics of β-D-glucosidase from a thermophilic fungus Thermomyces lanuginosus-SSBP. Biotechnol. Appl. Biochem. 30, 81–87.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 13, 265–275.
Lynd, L.R., P.J. Weimer, W.H. Zyl, and I.S. Pretorius. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577.
Mandels, G.R. 1953. Localization of carbohydrases at the surface of fungus spores by acid treatment. Exp. Cell Res. 5, 48–55.
Maras, M., A. DeBruyn, J. Schraml, P. Herdewijn, M. Claeyssens, W. Fiers, and R. Contreras. 1997. Structural characterization of Nlinked oligosaccharides from cellobiohydrolase secreted by filamentous fungi Trichoderma reesei Rut-C-30. Eur. J. Biochem. 245, 617–625.
Masheshwari, R., G. Bharadwaj, and M.K. Bhat. 2000. Thermophilic fungi: their physiology and enzymes. Microbiol. Mol. Biol. Rev. 64, 461–488.
Meldgaard, M. and I. Svendsen. 1994. Different effects of Nglycosylation on the thermostability of highly homologous bacterial (1,3-1,4)-β-glucananases secreted from yeast. Microbiology 140, 159–166.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 31, 426–428.
Nakkharat, P. and D. Haltrich. 2006. Purification and characterization of an intracellular enzyme with β-glucosidase and β-galactosidase activity from the thermophilic fungus Talaromyces thermophilus CBS 236.58. J. Biotechnol. 123, 304–313.
Nieves, R.A., C.I. Ehrman, W.S. Adney, R.T. Elander, and M.E. Himmel. 1998. Technical communication: Survey and analysis of commercial cellulose preparations suitable for biomass conversion to ethanol. World J. Microbiol. Biotechnol. 14, 301–304.
Osaki, H. and K. Yamada. 1991. Isolation of Streptomyces sp. producing glucose-tolerant β-glucosidases and properties of the enzymes. Agric. Biol. Chem. 55, 979–987.
Parry, N.J., D.E. Beever, E. Owen, I. Vandenberghe, J. Van Beeumen, and M.K. Bhat. 2001. Biochemical characterization and mechanisms of action of a thermostable β-glucosidase purified from Thermoascus aurantiacus. Biochem. J. 353, 117–127.
Peralta, R.M., M.K. Kadowaki, H.F. Terenzi, and J.A. Jorge. 1997. A highly thermostable β-glucosidase activity from the thermophilic fungus Humicola grisea var. thermoidea: purification and biochemical characterization. FEMS Microbiol. Lett. 146, 291–295.
Peralta, R.M., H.F. Terenzi, and J.A. Jorge. 1990. β-Glycosidase activities of Humicola grisea: biochemical and kinetic characterization of a multifunctional enzyme. Biochim. Biophys. Acta. 1033, 243–249.
Perez-Pons, J.A., X. Rebordosa, and E. Querol. 1995. Properties of a novel glucose-enhanced β-glucosidase purified from Streptomyces sp. (ATCC 11238). Biochim. Biophys. Acta. 1251, 145–153.
Polizeli, M.L.T.M., J.A. Jorge, and H.F. Terenzi. 1996. Effect of carbon source on the β-glucosidase system of the thermophilic fungus Humicola grisea. World J. Microbiol. Biotechnol. 12, 297–299.
Rao, U.S. and S.K. Murthy. 1988. Purification and characterization of a beta-glucosidase and endocellulase from Humicola insolens. Indian J. Biochem. Biophys. 25, 687–694.
Riou, C., J.M. Salmon, M.J. Vallier, Z. Günata, and P. Bare. 1998. Purification, characterization and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 64, 3607–3614.
Saha, B.C. and R.J. Bothast. 1996a. Glucose tolerant and thermophilic β-glucosidases from yeasts. Biotechnol. Lett. 18, 155–158.
Saha, B.C. and R.J. Bothast. 1996b. Production, purification and characterization of a highly glucose-tolerant novel β-glucosidase from Candida peltata. Appl. Environ. Microbiol. 62, 3165–3170.
Somera, A.F., M.G. Pereira, L.H.S. Guimarães, M.L.T.M. Polizeli, H.F. Terenzi, R.P.M. Furriel RPM, and J.A. Jorge. 2009. Effect of glycosylation on the biochemical properties of Aspergillus versicolor. J. Microbiol. 47, 270–276.
Sonia, K.G., B.S. Chadha, A.K. Badhan, H.S. Saini, and M.K. Bhat. 2008. Identification of glucose tolerant acid active β-glucosidases from thermophilic and thermotolerant fungi. World J. Microbiol. Biotechnol. 24, 599–604.
Stals, I., K. Sandra, B. Devreese, J. Van Beeumen, and M. Claeyssens. 2004a. Factors influencing glycosylation of Trichoderma reesei cellulases. II: N-glycosylation of Cel7A core protein isolated from different strains. Glycobiology 14, 725–737.
Stals, I., K. Sandra, S. Geysens, R. Contreras, J. Van Beeumen, and M. Claeyssens. 2004b. Factors influencing glycosylation of Trichoderma reesei cellulases. I: Postsecretorial changes of the O- and N-glycosylation pattern of Cel7A. Glycobiology 14, 713–724.
Varki, A. 1993. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3, 97–130.
Venturi, L.L., M.L.T.M. Polizeli, H.F. Terenzi, R.P.M. Furriel, and J.A. Jorge. 2002. Extracellular β-D-glucosidase from Chaetomium thermophilum var. coprophilum: production, purification and some biochemical properties. J. Basic Microbiol. 42, 55–66.
Weber, K. and M. Osborn. 1969. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 244, 4406–4412.
Wright, R.M., M.D. Yablonsky, Z.P. Shalita, A.K. Goyal, and D.E. Eveleigh. 1992. Cloning, characterization, nucleotide sequence of a gene encoding Microbispora bispora BglB, a thermostable β-glucosidase expressed in Escherichia coli. Appl. Environ. Microbiol. 58, 3455–3465.
Yang, S., Z. Jiang, Q. Yan, and H. Zhu. 2008. Characterization of a thermostable extracellular β-glucosidase with activities of exoglucanase and transglycosylation from Paecilomyces thermophila. J. Agric. Food Chem. 56, 602–608.
Yoon, J.J., K.Y. Kim, and C.J. Cha. 2008. Purification and characterization of thermostable β-glucosidase from the brown-rot basidiomycete Fomitopsis palustris grown on microcrystalline cellulose. J. Microbiol. 46, 51–55.
Zanoelo, F.F., M.L.T.M. Polizeli, H.F. Terenzi, and J.A. Jorge. 2004. β-Glucosidase activity from the thermophilic fungus Scytalidium thermophilum is stimulated by glucose and xylose. FEMS Microbiol. Lett. 240, 137–143.
Zhang, Y.H.P., M.E. Himmel, and J.R. Mielenz. 2006. Outlook for cellulase improvement: screening and selection strategies. Biotechnol. Adv. 24, 452–481.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nascimento, C.V., Souza, F.H.M., Masui, D.C. et al. Purification and biochemical properties of a glucose-stimulated β-D-glucosidase produced by Humicola grisea var. thermoidea grown on sugarcane bagasse. J Microbiol. 48, 53–62 (2010). https://doi.org/10.1007/s12275-009-0159-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-009-0159-x