Abstract
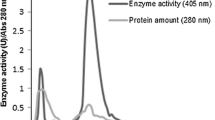
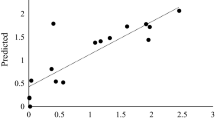
An extracellular β-glucosidase was purified 154-fold to electrophoretic homogeneity from the brown-rot basidiomycete Fomitopsis palustris grown on 2.0% microcrystalline cellulose. SDS-polyacrylamide gel electrophoresis gel gave a single protein band and the molecular mass of purified enzyme was estimated to be approximately 138 kDa. The amino acid sequences of the proteolytic fragments determined by nano-LC-MS/MS suggested that the protein has high homology with fungal β-glucosidases that belong to glycosyl hydrolase family 3. The K m s for p-nitorophenyl-β-d-glucoside (p-NPG) and cellobiose hydrolyses were 0.117 and 4.81 mM, and the K cat values were 721 and 101.8 per sec, respectively. The enzyme was competitively inhibited by both glucose (K i = 0.35 mM) and gluconolactone (K i 0.008 mM), when p-NPG was used as substrate. The optimal activity of the purified β-glucosidase was observed at pH 4.5 and 70°C. The F. palustris protein exhibited half-lives of 97 h at 55°C and 15 h at 65°C, indicating some degree of thermostability. The enzyme has high activity against p-NPG and cellobiose but has very little or no activity against p-nitrophenyl-β-lactoside, p-nitrophenyl-β-xyloside, p-nitrophenyl-α-arabinofuranoside, xylan, and carboxymethyl cellulose. Thus, our results revealed that the β-glucosidase from F. palustris can be classified as an aryl-β-glucosidase with cellobiase activity.
Similar content being viewed by others
References
Abe, S. and M. Takagi. 1991. Simultaneous saccharification and fermentation of cellulose to lactic acid. Biotechnol. Bioeng. 37, 93–96.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Cai, Y.J., J.A. Buswell, and S.T. Chang. 1998. β-Glucosidase components of the cellulolytic system of the edible straw mushroom, Volvariella volvacea. Enzyme Microb. Technol. 22, 122–129.
Cantarella, M., L. Cantarella, A. Gallifuoco, A. Spera, and F. Alfani. 2004. Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol. Prog. 20, 200–206.
Chen, H., M. Hayn, and H. Esterbauer. 1992. Purification and characterization of two extracellular beta-glucosidases from Trichoderma reesei. Biochim. Biophys. Acta. 1121, 54–60.
Chirico, W.J. and R.D. Brown, Jr. 1987. Purification and characterization of a beta-glucosidase from Trichoderma reesei. Eur. J. Biochem. 165, 343–351.
Claeyssens, M., H. Van Tilbeurgh, P. Tomme, T.M. Wood, and I. McCrae. 1989. Fungal cellulase systems. Comparison of the specificities of the cellobiohydrolases isolated from Penicillium pinophilum and Trichoderma reesei. Biochem. J. 261, 819–826.
Eriksson, K.-E.L., R.A. Blanchette, and P. Ander. 1990. Microbial and enzymatic degradation of wood and wood components. Springer Verlag, Berlin/Heidelberg, Germany.
Lee, J.-W., J.-Y. Park, K.-S. Gwak, B.-W. Koo, and I.-G. Choi. 2007. Characterization of β-glucosidase from brown rot fungus, Laetiporus sulphureus. Korean J. Wood Sci. Technol. 35, 100–108.
Lo, A.C., G. Willick, R. Bernier, and M. Desrochers. 1988. Purification and assay of β-glucosidase from Schizophyllum commune. Methods Enzymol. 160, 432–437.
Lymar, E.S., B. Li, and V. Renganathan. 1995. Purification and characterization of a cellulose-binding (beta)-glucosidase from cellulose-degrading cultures of Phanerochaete chrysosporium. Appl. Environ. Microbiol. 61, 2976–2980.
Magalhães, P.O., A. Ferraz, and A.F.M. Milaqres. 2006. Enzymatic properties of two beta-glucosidases from Ceriporiopsis subvermispora produced in biopulping conditions. J. Appl. Microbiol. 101, 480–486.
Martinez, G., L.F. Larrondo, N. Putnam, M.D.S. Gelpke, K. Huang, J. Chapman, K.G. Helfenbein, P. Ramaiya, J.C. Detter, F. Larimer, P.M. Coutinho, B. Henrissat, R. Berka, D. Cullen, and D. Rokhsar. 2004. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nature Biotechnol. 22, 1–6.
Saha, B.C., S.N. Freer, and R.J. Bothast. 1994. Production, purification, and properties of a thermostable beta-glucosidase from a color variant strain of Aureobasidium pullulans. Appl. Environ. Microbiol. 60, 3774–3780.
Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858.
Uzcategui, E., G. Johansson, B. Ek, and G. Pettersson. 1991. The 1,4-β-d-glucan glucanohydrolases from Phanerochaete chrysosporium. Reassessment of their significance in cellulose degradation mechanisms. J. Biotechnol. 21, 143–159.
Woodward, J., M. Lima, and N.E. Lee. 1982. The role of cellulose concentration in determining the degree of synergism in the hydrolysis of microcrystalline cellulose. Biochem. J. 255, 895–899.
Yoon, J.-J., C.-J. Cha, Y.-S. Kim, D.-W. Son, and Y.-K. Kim. 2007. The brown-rot basidiomycete Fomitopsis palustris has the endo-glucanases capable of degrading microcrystalline cellulose. J. Microbiol. Biotechnol. 17, 800–805.
Yoon, J.-J. and Y.-K. Kim. 2005. Degradation of crystalline cellulose by the brown-rot basidiomycete Fomitopsis palustris. J. Microbiol. 43, 487–492.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, JJ., Kim, KY. & Cha, CJ. Purification and characterization of thermostable β-glucosidase from the brown-rot basidiomycete Fomitopsis palustris grown on microcrystalline cellulose. J Microbiol. 46, 51–55 (2008). https://doi.org/10.1007/s12275-007-0230-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-007-0230-4