Abstract.
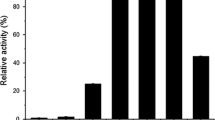
From Aspergillus tubingensis CBS 643.92 four distinct β-glucosidases (I–IV) were purified by a four-step purification procedure. SDS-PAGE revealed molecular masses of 131, 126, 54 and 54 kDa, respectively, and their isoelectric points were determined to be 4.2, 3.9, 3.7 and 3.6, respectively. The β-glucosidases exhibited high diversity with respect to pH and temperature optima and stability, as well as to substrate specificity and glucose tolerance. The major β-glucosidase (I) preferentially hydrolysed oligosaccharides. The acid-stable and heat-tolerant β-glucosidase II hydrolysed aryl and terpenyl β-D-glucosides as well as 1-O-trans-cinnamoyl β-D-glucoside. In contrast to β-glucosidases I and II, the minor β-glucosidases III and IV were found to be glucose-tolerant; inhibition constants of 470 and 600 mM, respectively, were determined.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received revision: 16 June 2000
Electronic Publication
Rights and permissions
About this article
Cite this article
Decker, .C., Visser, .J. & Schreier, .P. β-Glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Appl Microbiol Biotechnol 55, 157–163 (2001). https://doi.org/10.1007/s002530000462
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002530000462