Abstract
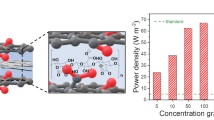
Reverse electrodialysis (RED), based on ion-selective membranes, is one of the most promising technologies for capturing osmotic energy. As key elements of the RED system, ion-selective membranes must meet the crucial demands of mechanical stability, anti-fouling characteristics, easy fabrication, and high power density; however, this still remains a challenge. In this study, we demonstrated a large-scale, mechanically stable, and high-porosity membrane obtained by combining carbon nanomaterials and hyperbranched polyethyleneimine (h-PEI), thereby achieving a high power density of 5.0 W·m−2 with seawater and river water. Carbon nanofibers (CNFs) were subsequently bridged with graphene and h-PEI to strengthen the interaction between the CNFs, reduce the channel size and increase the space charge density, mechanical strength, and toughness. The large-scale and mechanically stable membrane fabricated using the modified CNFs exhibited anion selectivity and high ionic conductivity, thereby achieving a high-performance osmotic energy conversion. Furthermore, the anti-fouling property of the membrane was confirmed by the stability of the osmotic energy conversion in a solution with algae, which can be attributed to the high porosity of carbon nanomaterials. This economic and convenient method for the ion-selective membrane preparation is believed to be promising for large-scale osmotic energy harvesting.

Similar content being viewed by others
References
Armaroli, N.; Balzani, V. The future of energy supply: Challenges and opportunities. Angew. Chem., Int. Ed. 2007, 46, 52–66.
Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M. C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Sustainable development goals for people and planet. Nature 2013, 495, 305–307.
Aricò, A. S.; Bruce, P.; Scrosati, B.; Tarascon, J. M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377.
Crittenden, J. C.; White, H. S. Harnessing energy for a sustainable world. J. Am. Chem. Soc. 2010, 132, 4503–4505.
Siria, A.; Bocquet, M. L.; Bocquet, L. New avenues for the large-scale harvesting of blue energy. Nat. Rev. Chem. 2017, 1, 0091.
Yip, N. Y.; Brogioli, D.; Hamelers, H. V. M.; Nijmeijer, K. Salinity gradients for sustainable energy: Primer, progress, and prospects. Environ. Sci. Technol. 2016, 50, 12072–12094.
Wang, Z. L.; Jiang, T.; Xu, L. Toward the blue energy dream by triboelectric nanogenerator networks. Nano Energy 2017, 39, 9–23.
La Mantia, F.; Pasta, M.; Deshazer, H. D.; Logan, B. E.; Cui, Y. Batteries for efficient energy extraction from a water salinity difference. Nano Lett. 2011, 11, 1810–1813.
Tian, H. L.; Wang, Y.; Pei, Y. S.; Crittenden, J. C. Unique applications and improvements of reverse electrodialysis: A review and outlook. Appl. Energy 2020, 262, 114482.
Tristán, C.; Rumayor, M.; Dominguez-Ramos, A.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Life cycle assessment of salinity gradient energy recovery by reverse electrodialysis in a seawater reverse osmosis desalination plant. Sustainable Energy Fuels 2020, 4, 4273–4284.
Logan, B. E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319.
Long, R.; Li, M. L.; Chen, X.; Liu, Z. C.; Liu, W. Synergy analysis for ion selectivity in nanofluidic salinity gradient energy harvesting. Int. J. Heat Mass Transf. 2021, 171, 121126.
Pakulski, D.; Czepa, W.; Del Buffa, S.; Ciesielski, A.; Samorì, P. Atom-thick membranes for water purification and blue energy harvesting. Adv. Funct. Mater. 2020, 30, 1902394.
Zhou, Y. H.; Jiang, L. Bioinspired nanoporous membrane for salinity gradient energy harvesting. Joule 2020, 4, 2244–2248.
Vermaas, D. A.; Saakes, M.; Nijmeijer, K. Doubled power density from salinity gradients at reduced intermembrane distance. Environ. Sci. Technol. 2011, 45, 7089–7095.
Tufa, R. A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Goulão Crespo, J.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331.
Liu, Y. Q.; Ping, J. F.; Ying, Y. B. Anion-selective layered double hydroxide composites-based osmotic energy conversion for real-time nutrient solution detection. Adv. Sci. 2022, 9, 2103696.
Hao, J. L.; Yang, T.; He, X. L.; Tang, H. Y.; Sui, X. Hierarchical nanochannels based on rod-coil block copolymer for ion transport and energy conversion. Giant 2021, 5, 100049.
Hao, J. L.; Wang, W. J.; Zhao, J. W.; Che, H. L.; Chen, L.; Sui, X. Construction and application of bioinspired nanochannels based on two-dimensional materials. Chin. Chem. Lett. 2022, 33, 2291–2300.
Xin, W. W.; Jiang, L.; Wen, L. P. Two-dimensional nanofluidic membranes toward harvesting salinity gradient power. Acc. Chem. Res. 2021, 54, 4154–4165.
Sui, X.; Zhang, Z.; Zhang, Z. Y.; Wang, Z. W.; Li, C.; Yuan, H.; Gao, L. C.; Wen, L. P.; Fan, X.; Yang, L. J. et al. Biomimetic nanofluidic diode composed of dual amphoteric channels maintains rectification direction over a wide pH range. Angew. Chem., Int. Ed. 2016, 55, 13056–13060.
Ma, H.; Wang, S.; Yu, B.; Sui, X.; Shen, Y. Q.; Cong, H. L. Bioinspired nanochannels based on polymeric membranes. Sci. China Mater. 2021, 64, 1320–1342.
Zhang, Z.; Yang, S.; Zhang, P. P.; Zhang, J.; Chen, G. B.; Feng, X. L. Mechanically strong MXene/Kevlar nanofiber composite membranes as high-performance nanofluidic osmotic power generators. Nat. Commun. 2019, 10, 2920.
Sun, J.; Li, Q.; Zhu, H.; Liu, Z. N.; Lin, K.; Wang, N.; Zhang, Q. H.; Gu, L.; Deng, J. X.; Chen, J. et al. Negative-pressure-induced large polarization in nanosized PbTiO3. Adv. Mater. 2020, 32, 2002968.
Güler, E.; Elizen, R.; Vermaas, D. A.; Saakes, M.; Nijmeijer, K. Performance-determining membrane properties in reverse electrodialysis. J. Membr. Sci. 2013, 446, 266–276.
Xie, L.; Zhou, S.; Liu, J. R.; Qiu, B. L.; Liu, T. Y.; Liang, Q. R.; Zheng, X. Z.; Li, B.; Zeng, J.; Yan, M. et al. Sequential superassembly of nanofiber arrays to carbonaceous ordered mesoporous nanowires and their heterostructure membranes for osmotic energy conversion. J. Am. Chem. Soc. 2021, 143, 6922–6932.
Zhou, S.; Xie, L.; Li, X. F.; Huang, Y. N.; Zhang, L. P.; Liang, Q. R.; Yan, M.; Zeng, J.; Qiu, B. L.; Liu, T. Y. et al. Interfacial super-assembly of ordered mesoporous carbon-silica/AAO hybrid membrane with enhanced permselectivity for temperature- and pH-sensitive smart ion transport. Angew. Chem., Int. Ed. 2021, 60, 26167–26176.
Fu, L.; Merabia, S.; Joly, L. Understanding fast and robust thermoosmotic flows through carbon nanotube membranes: Thermodynamics meets hydrodynamics. J. Phys. Chem. Lett. 2018, 9, 2086–2092.
Sun, T. Y.; Yang, L. P.; Tang, J. B.; Li, N. B.; Chen, J. L.; Shen, A. Q.; Shao, Y.; Zhang, Y. F.; Liu, H.; Xue, G. B. Flocculating-filtration-processed mesoporous structure in laminar ion-selective membrane for osmosis energy conversion and desalination. Chem. Eng. J. 2022, 437, 135484.
Zhang, Z. K.; Shen, W. H.; Lin, L. X.; Wang, M.; Li, N.; Zheng, Z. F.; Liu, F.; Cao, L. X. Vertically transported graphene oxide for highperformance osmotic energy conversion. Adv. Sci. 2020, 7, 2000286.
Joly, L.; Meißner, R. H.; Iannuzzi, M.; Tocci, G. Osmotic transport at the aqueous graphene and hBN interfaces: Scaling laws from a unified, first-principles description. ACS Nano 2021, 15, 15249–15258.
Ji, J. Z.; Kang, Q.; Zhou, Y.; Feng, Y. P.; Chen, X.; Yuan, J. Y.; Guo, W.; Wei, Y.; Jiang, L. Osmotic power generation with positively and negatively charged 2D nanofluidic membrane pairs. Adv. Funct. Mater. 2017, 27, 1603623.
Hong, S.; Constans, C.; Surmani Martins, M. V.; Seow, Y. C.; Guevara Carrió, J. A.; Garaj, S. Scalable graphene-based membranes for ionic sieving with ultrahigh charge selectivity. Nano Lett. 2017, 17, 728–732.
Raidongia, K.; Huang, J. X. Nanofluidic ion transport through reconstructed layered materials. J. Am. Chem. Soc. 2012, 134, 16528–16531.
Liu, W. H.; Lin, Y. X.; Song, L. X.; Xiong, J. Research progress of flexible carbon based nanofibers films. Silk 2020, 57, 1–8.
Yu, Q. Q.; Chen, G.; Wang, Q. F.; Chong, L.; Wang, Z. Y.; Wu, Z. Q.; Wang, Z.; Huang, C. X. Research progress on properties and applications of PAN-based carbon nanofiber films based on electrospinning technology. Eng. Plast. Appl. 2021, 49, 166–174.
Sparreboom, W.; van den Berg, A.; Eijkel, J. C. T. Principles and applications of nanofluidic transport. Nat. Nanotechnol. 2009, 4, 713–720.
Luo, Q. X.; Liu, P.; Fu, L.; Hu, Y. H.; Yang, L. S.; Wu, W. W.; Kong, X. Y.; Jiang, L.; Wen, L. P. Engineered cellulose nanofiber membranes with ultrathin low-dimensional carbon material layers for photothermal-enhanced osmotic energy conversion. ACS Appl. Mater. Interfaces 2022, 14, 13223–13230.
Zeng, J.; Ji, X. X.; Ma, Y. H.; Zhang, Z. X.; Wang, S. G.; Ren, Z. H.; Zhi, C. Y.; Yu, J. 3D graphene fibers grown by thermal chemical vapor deposition. Adv. Mater. 2018, 30, 1705380.
Zhang, Z.; Sui, X.; Li, P.; Xie, G. H.; Kong, X. Y.; Xiao, K.; Gao, L. C.; Wen, L. P.; Jiang, L. Ultrathin and ion-selective Janus membranes for high-performance osmotic energy conversion. J. Am. Chem. Soc. 2017, 139, 8905–8914.
Cheng, P.; Chen, S.; Li, X.; Xu, Y. L.; Xu, F.; Ragauskas, A. J. Tree-inspired lignin microrods-based composite heterogeneous nanochannels for ion transport and osmotic energy harvesting. Energy Convers. Manag. 2022, 255, 115321.
Bian, G. S.; Pan, N.; Luan, Z. H.; Sui, X.; Fan, W. X.; Xia, Y. Z.; Sui, K. Y.; Jiang, L. Anti-swelling gradient polyelectrolyte hydrogel membranes as high-performance osmotic energy generators. Angew. Chem., Int. Ed. 2021, 60, 20294–20300.
Ulbricht, M.; Belfort, G. Surface modification of ultrafiltration membranes by low temperature plasma II. Graft polymerization onto polyacrylonitrile and polysulfone.. J. Membr. Sci. 1996, 111, 193–215.
Harada, R.; Kojima, T. A porphyrin nanochannel: Formation of cationic channels by a protonated saddle-distorted porphyrin and its inclusion behavior. Chem. Commun. 2005, 716–718.
Liu, Q.; Wen, L. P.; Xiao, K.; Lu, H.; Zhang, Z.; Xie, G. H.; Kong, X. Y.; Bo, Z. S.; Jiang, L. A biomimetic voltage-gated chloride nanochannel. Adv. Mater. 2016, 28, 3181–3186.
Freger, V.; Bason, S. Characterization of ion transport in thin films using electrochemical impedance spectroscopy: I. Principles and theory. J. Membr. Sci. 2007, 302, 1–9.
Zhang, Z.; He, L.; Zhu, C. C.; Qian, Y. C.; Wen, L. P.; Jiang, L. Improved osmotic energy conversion in heterogeneous membrane boosted by three-dimensional hydrogel interface. Nat. Commun. 2020, 11, 875.
Schoch, R. B.; Han, J.; Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 2008, 80, 839–883.
Constantin, D.; Siwy, Z. S. Poisson-Nernst-Planck model of ion current rectification through a nanofluidic diode. Phys. Rev. E 2007, 76, 041202.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 22005162), the Natural Science Foundation of Shandong Province (No. ZR2020QE093) and the Special Financial Aid to Post-doctor Research Fellow (No. 2020T130330).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, W., Hao, J., Sun, Q. et al. Carbon nanofibers membrane bridged with graphene nanosheet and hyperbranched polymer for high-performance osmotic energy harvesting. Nano Res. 16, 1205–1211 (2023). https://doi.org/10.1007/s12274-022-4634-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4634-6