Abstract
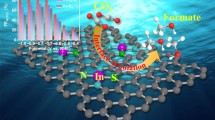
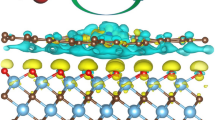
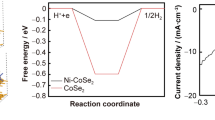
CoS2 is considered to be a promising electrocatalyst for hydrogen evolution reaction (HER). However, its further widespread applications are hampered by the unsatisfactory activity due to relatively high chemisorption energy for hydrogen atom. Herein, theoretical predictions of first-principles calculations reveal that the introduction of a Cl-terminated MXenes-Ti3CNCl2 can significantly reduce the HER potential of CoS2-based materials and the Ti3CNCl2@CoS2 core-shell nanostructure has Gibbs free energy of hydrogen adsorption (∣ΔGH∣) close to zero, much lower than that of the pristine CoS2 and Ti3CNCl2. Inspired by the theoretical predictions, we have successfully fabricated a unique Ti3CNCl2@CoS2 core-shell nanostructure by ingeniously coupling CoS2 with a Cl-terminated MXenes-Ti3CNCl2. Interface-charge transfer between CoS2 and Ti3CNCl2 results in a higher degree of electronic localization and a formation of chemical bonding. Thus, the Ti3CNCl2@CoS2 core-shell nanostructure achieves a significant enhancement in HER activity compared to pristine CoS2 and Ti3CNCl2. Theoretical calculations further confirm that the partial density of states of CoS2 after hybridization becomes more non-localized, and easier to interact with hydrogen ions, thus boosting HER performance. In this work, the success of oriented experimental fabrication of high-efficiency Ti3CNCl2@CoS2 electrocatalysts guided by theoretical predictions provides a powerful lead for the further strategic design and fabrication of efficient HER electrocatalysts.

Similar content being viewed by others
References
Lin, L. X.; Sherrell, P.; Liu, Y. Q.; Lei, W.; Zhang, S. W.; Zhang, H. J.; Wallace, G. G.; Chen, J. Engineered 2D transition metal dichalcogenides—a vision of viable hydrogen evolution reaction catalysis. Adv. Energy Mater. 2020, 10, 1903870.
Ou, G.; Fan, P. X.; Ke, X. X.; Xu, Y. S.; Huang, K.; Wei, H. H.; Yu, W.; Zhang, H. J.; Zhong, M. L.; Wu, H. et al. Defective molybdenum sulfide quantum dots as highly active hydrogen evolution electrocatalysts. Nano Res. 2018, 11, 751–761.
Zhu, Y. L.; Lin, Q.; Zhong, Y. J.; Tahini, H. A.; Shao, Z. P.; Wang, H. T. Metal oxide-based materials as an emerging family of hydrogen evolution electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392.
Li, B.; Wu, Y.; Li, N.; Chen, X. Z.; Zeng, X. B.; Arramel; Zhao, X. J.; Jiang, J. Z. Single-metal atoms supported on MBenes for robust electrochemical hydrogen evolution. ACS Appl. Mater. Interfaces 2020, 12, 9261–9267.
Zeng, Z. L.; Chen, X. Z.; Weng, K. Y.; Wu, Y.; Zhang, P.; Jiang, J. Z.; Li, N. Computational screening study of double transition metal carbonitrides M′2M″CNO2-MXene as catalysts for hydrogen evolution reaction. npj Comput. Mater. 2021, 7, 80.
Fu, Q.; Han, J. C.; Wang, X. J.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W. W.; Liu, S. W.; Gao, T. L.; Zhang, Z. H. et al. 2D transition metal dichalcogenides: Design, modulation, and challenges in electrocatalysis. Adv. Mater. 2021, 33, 1907818.
Zhang, J. Q.; Zhao, Y. F.; Guo, X.; Chen, C.; Dong, C. L.; Liu, R. S.; Han, C. P.; Li, Y. D.; Gogotsi, Y.; Wang, G. X. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992.
Liu, S. W.; Cai, J. Y.; Wang, G. M. Two-dimensional MoS2 for hydrogen evolution reaction catalysis: The electronic structure regulation. Nano Res. 2021, 14, 1985–2002.
Wang, Y.; Zheng, X. B.; Wang, D. S. Design concept for electrocatalysts. Nano Res. 2022, 15, 1730–1752.
Yang, J. R.; Li, W. H.; Tan, S. D.; Xu, K. N.; Wang, Y.; Wang, D. S.; Li, Y. D. The electronic metal-support interaction directing the design of single atomic site catalysts: Achieving high efficiency towards hydrogen evolution. Angew. Chem., Int. Ed. 2021, 60, 19085–19091.
Zhang, J. Y.; Xiao, W.; Xi, P. X.; Xi, S. B.; Du, Y. H.; Gao, D. Q.; Ding, J. Activating and optimizing activity of CoS2 for hydrogen evolution reaction through the synergic effect of N dopants and S vacancies. ACS Energy Lett. 2017, 2, 1022–1028.
Borthakur, P.; Boruah, P. K.; Das, M. R.; Ibrahim, M. M.; Altalhi, T.; El-Sheshtawy, H. S.; Szunerits, S.; Boukherroub, R.; Amin, M. A. CoS2 nanoparticles supported on rGO, g-C3N4, BCN, MoS2, and WS2 two-dimensional nanosheets with excellent electrocatalytic performance for overall water splitting: Electrochemical studies and DFT calculations. ACS Appl. Energy Mater. 2021, 4, 1269–1285.
Zhu, L. F.; Liu, L. J.; Huang, G. M.; Zhao, Q. Hydrogen evolution over N-doped CoS2 nanosheets enhanced by superaerophobicity and electronic modulation. Appl. Surf. Sci. 2020, 504, 144490.
Zhang, Y. Y.; Zhang, X.; Wu, Z. Y.; Zhang, B. B.; Zhang, Y.; Jiang, W. J.; Yang, Y. G.; Kong, Q. H.; Hu, J. S. Fe/P dual doping boosts the activity and durability of CoS2 polycrystalline nanowires for hydrogen evolution. J. Mater. Chem. A 2019, 7, 5195–5200.
Faber, M. S.; Dziedzic, R.; Lukowski, M. A.; Kaiser, N. S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061.
Zhang, H. C.; Li, Y. J.; Zhang, G. X.; Wan, P. B.; Xu, T. H.; Wu, X. C.; Sun, X. M. Highly crystallized cubic cattierite CoS2 for electrochemically hydrogen evolution over wide pH range from 0 to 14. Electrochim. Acta 2014, 148, 170–174.
Zhang, J. Y.; Liu, Y. C.; Sun, C. Q.; Xi, P. X.; Peng, S. L.; Gao, D. Q.; Xue, D. S. Accelerated hydrogen evolution reaction in CoS2 by transition-metal doping. ACS Energy Lett. 2018, 3, 779–786.
Chen, P. Z.; Zhou, T. P.; Chen, M. L.; Tong, Y.; Zhang, N.; Peng, X.; Chu, W. S.; Wu, X. J.; Wu, C. Z.; Xie, Y. Enhanced catalytic activity in nitrogen-anion modified metallic cobalt disulfide porous nanowire arrays for hydrogen evolution. ACS Catal. 2017, 7, 7405–7411.
Li, J. Y.; Xia, Z. M.; Zhou, X. M.; Qin, Y. B.; Ma, Y. Y.; Qu, Y. Q. Quaternary pyrite-structured nickel/cobalt phosphosulfide nanowires on carbon cloth as efficient and robust electrodes for water electrolysis. Nano Res. 2017, 10, 814–825.
Peng, S. J.; Li, L. L.; Han, X. P.; Sun, W. P.; Srinivasan, M.; Mhaisalkar, S. G.; Cheng, F. Y.; Yan, Q. Y.; Chen, J.; Ramakrishna, S. Cobalt sulfide nanosheet/graphene/carbon nanotube nanocomposites as flexible electrodes for hydrogen evolution. Angew. Chem., Int. Ed. 2014, 126, 12802–12807.
Ahn, S.; Yang, J. U.; Lim, H.; Shin, H. S. Selective synthesis of pure cobalt disulfide on reduced graphene oxide sheets and its high electrocatalytic activity for hydrogen evolution reaction. Nano Converg. 2016, 3, 5.
Feng, Y. Y.; Zhang, T.; Zhang, J. H.; Fan, H.; He, C.; Song, J. X. 3D 1T-MoS2/CoS2 heterostructure via interface engineering for ultrafast hydrogen evolution reaction. Small 2020, 16, 2002850.
Zang, Y.; Yang, B. P.; Li, A.; Liao, C. G.; Chen, G.; Liu, M.; Liu, X. H.; Ma, R. Z.; Zhang, N. Tuning interfacial active sites over porous Mo2N-supported cobalt sulfides for efficient hydrogen evolution reactions in acid and alkaline electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 41573–41583.
Liu, Y. W.; Li, J.; Huang, W. T.; Zhang, Y.; Wang, M. J.; Gao, X. S.; Wang, X.; Jin, M. L.; Hou, Z. P.; Zhou, G. F. et al. Surface-induced 2D/1D heterostructured growth of ReS2/CoS2 for high-performance electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 33586–33594.
Tong, Y.; Sun, Q.; Chen, P. Z.; Chen, L.; Fei, Z. F.; Dyson, P. J. Nitrogen-incorporated cobalt sulfide/graphene hybrid catalysts for overall water splitting. ChemSusChem 2020, 13, 5112–5118.
Seh, Z. W.; Fredrickson, K. D.; Anasori, B.; Kibsgaard, J.; Strickler, A. L.; Lukatskaya, M. R.; Gogotsi, Y.; Jaramillo, T. F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594.
Gao, G. P.; O’Mullane, A. P.; Du, A. J. 2D MXenes: A new family of promising catalysts for the hydrogen evolution reaction. ACS Catal. 2017, 7, 494–500.
Li, S.; Tuo, P.; Xie, J. F.; Zhang, X. D.; Xu, X. G.; Bao, J.; Pan, B. C.; Xie, Y. Ultrathin MXene nanosheets with rich fluorine termination groups realizing efficient electrocatalytic hydrogen evolution. Nano Energy 2018, 47, 512–518.
Bai, S. S.; Yang, M. Q.; Jiang, J. Z.; He, X. M.; Zou, J.; Xiong, Z. G.; Liao, G. D.; Liu, S. Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. npj 2D Mater. Appl. 2021, 5, 78.
Ding, B.; Ong, W. J.; Jiang, J. Z.; Chen, X. Z.; Li, N. Uncovering the electrochemical mechanisms for hydrogen evolution reaction of heteroatom doped M2C MXene (M = Ti, Mo). Appl. Surf. Sci. 2020, 500, 143987.
Liu, J. P.; Liu, Y. Z.; Xu, D. Y.; Zhu, Y. Z.; Peng, W. C.; Li, Y.; Zhang, F. B.; Fan, X. B. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B 2019, 241, 89–94.
Wang, Z. G.; Xu, W. Q.; Yu, K.; Feng, Y.; Zhu, Z. Q. 2D heterogeneous vanadium compound interfacial modulation enhanced synergistic catalytic hydrogen evolution for full pH range seawater splitting. Nanoscale 2020, 12, 6176–6187.
Jiang, H. M.; Wang, Z. G.; Yang, Q.; Tan, L. X.; Dong, L. C.; Dong, M. D. Ultrathin Ti3C2Tx (MXene) nanosheet-wrapped NiSe2 octahedral crystal for enhanced supercapacitor performance and synergetic electrocatalytic water splitting. Nano-Micro Lett. 2019, 11, 31.
Kuang, P. Y.; He, M.; Zhu, B. C.; Yu, J. G.; Fan, K.; Jaroniec, M. 0D/2D NiS2/V-MXene composite for electrocatalytic H2 evolution. J. Catal. 2019, 375, 8–20.
Li, N.; Zhang, Y. F.; Jia, M. L.; Lv, X. D.; Li, X. T.; Li, R.; Ding, X. Q.; Zheng, Y. Z.; Tao, X. 1T/2H MoSe2-on-MXene heterostructure as bifunctional electrocatalyst for efficient overall water splitting. Electrochim. Acta 2019, 326, 134976.
Han, S. L.; Chen, Y.; Hao, Y. N.; Xie, Y. Y.; Xie, D. Y.; Chen, Y.; Xiong, Y. X.; He, Z. Y.; Hu, F.; Li, L. L. et al. Multi-dimensional hierarchical CoS2@MXene as trifunctional electrocatalysts for zinc-air batteries and overall water splitting. Sci. China Mater. 2021, 64, 1127–1138.
Liu, S. L.; Lin, Z. S.; Wan, R. D.; Liu, Y. G.; Liu, Z.; Zhang, S. D.; Zhang, X. F.; Tang, Z. H.; Lu, X. X.; Tian, Y. Cobalt phosphide supported by two-dimensional molybdenum carbide (MXene) for the hydrogen evolution reaction, oxygen evolution reaction, and overall water splitting. J. Mater. Chem. A 2021, 9, 21259–21269.
Xiu, L. Y.; Wang, Z. Y.; Yu, M. Z.; Wu, X. H.; Qiu, J. S. Aggregation-resistant 3D MXene-based architecture as efficient bifunctional electrocatalyst for overall water splitting. ACS Nano 2018, 12, 8017–8028.
Yan, L.; Zhang, B. Rose-like, ruthenium-modified cobalt nitride nanoflowers grown in situ on an MXene matrix for efficient and stable water electrolysis. J. Mater. Chem. A 2021, 9, 20758–20765.
Wang, H.; Lin, Y. P.; Liu, S. Y.; Li, J. M.; Bu, L. M.; Chen, J. M.; Xiao, X.; Choi, J. H.; Gao, L. J.; Lee, J. M. Confined growth of pyridinic N-Mo2C sites on MXenes for hydrogen evolution. J. Mater. Chem. A 2020, 8, 7109–7116.
Wang, J. Y.; He, P. L.; Shen, Y. L.; Dai, L. X.; Li, Z.; Wu, Y.; An, C. H. FeNi nanoparticles on Mo2TiC2Tx MXene@nickel foam as robust electrocatalysts for overall water splitting. Nano Res. 2021, 14, 3474–3481.
Li, Z.; Qi, Z. Y.; Wang, S. W.; Ma, T.; Zhou, L.; Wu, Z. W.; Luan, X. C.; Lin, F. Y.; Chen, M. D.; Miller, J. T. et al. In situ formed Pt3Ti nanoparticles on a two-dimensional transition metal carbide (MXene) used as efficient catalysts for hydrogen evolution reactions. Nano Lett. 2019, 19, 5102–5108.
Zhu, X. D.; Xie, Y.; Liu, Y. T. Exploring the synergy of 2D MXene-supported black phosphorus quantum dots in hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2018, 6, 21255–21260.
Jiang, Y. N.; Sun, T.; Xie, X.; Jiang, W.; Li, J.; Tian, B. B.; Su, C. L. Oxygen-functionalized ultrathin Ti3C2Tx MXene for enhanced electrocatalytic hydrogen evolution. ChemSusChem 2019, 12, 1368–1373.
Kamysbayev, V.; Filatov, A. S.; Hu, H. C.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R. F.; Talapin, D. V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983.
Zou, J.; Wu, J.; Wang, Y. Z.; Deng, F. X.; Jiang, J. Z.; Zhang, Y. Z.; Liu, S.; Li, N.; Zhang, H.; Yu, J. G. et al. Additive-mediated intercalation and surface modification of MXenes. Chem. Soc. Rev., in press, https://doi.org/10.1039/D0CS01487G
Li, Y. B.; Shao, H.; Lin, Z. F.; Lu, J.; Liu, L. Y.; Duployer, B.; Persson, P. O. A.; Eklund, P.; Hultman, L.; Li, M. et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899.
Kresse, G., Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Perdew, J. P.; Chevary, J. A.; Vosko, S. H.; Jackson, K. A.; Pederson, M. R.; Singh, D. J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687.
Monkhorst, H. J.; Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799.
Momma, K.; Izumi, F. VESTA 3 for Three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276.
Jiang, J. Z.; Zou, Y. L.; Arramel; Li, F. Y.; Wang, J. M.; Zou, J.; Li, N. Intercalation engineering of MXenes towards highly efficient photo(electrocatalytic) hydrogen evolution reactions. J. Mater. Chem. A 2021, 9, 24195–24214.
Wang, F.; Niu, S. W.; Liang, X. Q.; Wang, G. M.; Chen, M. H. Phosphorus incorporation activates the basal plane of tungsten disulfide for efficient hydrogen evolution catalysis. Nano Res., in press, DOI: https://doi.org/10.1007/s12274-021-3873-2.
Liu, T.; Mai, X. M.; Chen, H. J.; Ren, J.; Liu, Z. T.; Li, Y. X.; Gao, L. N.; Wang, N.; Zhang, J. X.; He, H. C. et al. Carbon nanotube aerogel-CoS2 hybrid catalytic counter electrodes for enhanced photovoltaic performance dye-sensitized solar cells. Nanoscale 2018, 10, 4194–4201.
Xie, W. J.; Liu, K.; Shi, G. D.; Fu, X. L.; Chen, X. J.; Fan, Z. X.; Liu, M.; Yuan, M. J.; Wang, M. CoS2 nanowires supported graphdiyne for highly efficient hydrogen evolution reaction. J. Energy Chem. 2021, 60, 272–278.
Zhu, J. W.; Wang, M.; Lyu, M. Q.; Jiao, Y. L.; Du, A. J.; Luo, B.; Gentle, I.; Wang, L. Z. Two-dimensional titanium carbonitride MXene for high-performance sodium ion batteries. ACS Appl. Nano Mater. 2018, 1, 6854–6863.
Wang, K. F.; Chu, W. S.; Li, H.; Chen, Y. J.; Cai, Y. L.; Liu, H. Z. Ferromagnetic Ti3CNCl2-decorated RGO aerogel: From 3D interconnecting conductive network construction to ultra-broadband microwave absorber with thermal insulation property. J. Colloid Interface Sci. 2021, 604, 402–414.
Zhang, G.; Wang, P.; Lu, W. T.; Wang, C. Y.; Li, Y. K.; Ding, C.; Gu, J. J.; Zheng, X. S.; Gao, F. F. Co nanoparticles/Co, N, S tri-doped graphene templated from in-situ-formed Co, S Co-doped g-C3N4 as an active bifunctional electrocatalyst for overall water splitting. ACS Appl. Mater. Interfaces 2017, 9, 28566–28576.
Jung, J. Y.; Hong, Y. L.; Kim, J. G.; Kim, M. J.; Kim, Y. K.; Kim, N. D. New insight of tailor-made graphene oxide for the formation of atomic Co-N sites toward hydrogen evolution reaction. Appl. Surf. Sci. 2021, 563, 150254.
Wei, R. P.; Dong, Y. T.; Zhang, Y. Y.; Kang, X. Y.; Sheng, X.; Zhang, J. M. Hollow cubic MnS-CoS2-NC@NC designed by two kinds of nitrogen-doped carbon strategy for sodium ion batteries with ultraordinary rate and cycling performance. Nano Res., in press, https://doi.org/10.1007/s12274-021-3973-z.
Hou, Y.; Qiu, M.; Zhang, T.; Ma, J.; Liu, S. H.; Zhuang, X. D.; Yuan, C.; Feng, X. L. Efficient electrochemical and photoelectrochemical water splitting by a 3D nanostructured carbon supported on flexible exfoliated graphene foil. Adv. Mater. 2017, 29, 1604480.
Pu, Y. Y.; Celorrio, V.; Stockmann, J. M.; Sobol, O.; Sun, Z. Z.; Wang, W.; Lawrence, M. J.; Radnik, J.; Russell, A. E.; Hodoroaba, V. D. et al. Surface galvanic formation of Co-OH on Birnessite and its catalytic activity for the oxygen evolution reaction. J. Catal. 2021, 396, 304–314.
Li, M.; Lu, J.; Luo, K.; Li, Y. B.; Chang, K. K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P. et al. Element replacement approach by reaction with lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737.
Ma, B.; Yang, Z. C.; Chen, Y. T.; Yuan, Z. H. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2019, 12, 375–380.
Zhu, J.; Hu, L. S.; Zhao, P. X.; Lee, L. Y. S.; Wong, K. Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918.
Yu, L.; Zhou, H. Q.; Sun, J. Y.; Qin, F.; Yu, F.; Bao, J. M.; Yu, Y.; Chen, S.; Ren, Z. F. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 62004143), the Central Government Guided Local Science and Technology Development Special Fund Project (No. 2020ZYYD033), the Natural Science Foundation of Hubei Province (No. 2021CFB133), the Opening Fund of Key Laboratory of Rare Mineral, Ministry of Natural Resources (No. KLRM-KF 202005), the Opening Fund of Key Laboratory for Green Chemical Process of Ministry of Education of Wuhan Institute of Technology (No. GCP202101), and the Innovation Project of Engineering Research Center of Phosphorus Resources Development and Utilization of Ministry of Education (No. LCX2021003). This work was dedicated to celebrating the 50th anniversary of Wuhan Institute of Technology.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4276_MOESM1_ESM.pdf
Strategic design and fabrication of MXenes-Ti3CNCl2@CoS2 core-shell nanostructure for high-efficiency hydrogen evolution
Rights and permissions
About this article
Cite this article
Jiang, J., Bai, S., Yang, M. et al. Strategic design and fabrication of MXenes-Ti3CNCl2@CoS2 core-shell nanostructure for high-efficiency hydrogen evolution. Nano Res. 15, 5977–5986 (2022). https://doi.org/10.1007/s12274-022-4276-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4276-8