Abstract
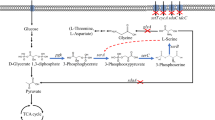
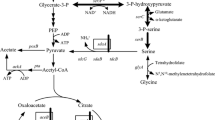
In E. coli, glyA encodes for serine hydroxymethyltransferase (SHMT), which converts L-serine to glycine. When engineering L-serine-producing strains, it is therefore favorable to inactivate glyA to prevent L-serine degradation. However, most glyA knockout strains exhibit slow cell growth because of the resulting lack of glycine and C1 units. To overcome this problem, we overexpressed the gcvTHP genes of the glycine cleavage system (GCV), to increase the C1 supply before glyA was knocked out. Subsequently, the kbl and tdh genes were overexpressed to provide additional glycine via the L-threonine degradation pathway, thus restoring normal cell growth independent of glycine addition. Finally, the plasmid pPK10 was introduced to overexpress pgk, serA Δ197, serC and serB, and the resulting strain E4G2 (pPK10) accumulated 266.3 mg/L of L-serine in a semi-defined medium without adding glycine, which was 3.18-fold higher than the production achieved by the control strain E3 (pPK10). This strategy can accordingly be applied to disrupt the L-serine degradation pathway in industrial production strains without causing negative side-effects, ultimately making L-serine production more efficient.
Similar content being viewed by others
References
Hsiao, H. Y. and T. Wei (1986) Enzymatic production of L-serine with a feedback control system for formaldehyde addition. Biotechnol. Bioeng. 28: 1510–1518.
Hagishita, T., T. Yoshida, Y. Izumi, and T. Mitsunaga (1996) Efficient l-serine production from methanol and glycine by resting cells of Methylobacterium sp. strain MN43. Biosci. Biotechnol. Biochem. 60: 1604–1607.
Jiang, W., B. Xia, and Z. Liu (2013) A serine hydroxymethyltransferase from marine bacterium Shewanella algae: Isolation, purification, characterization and l-serine production. Microbiol. Res. 168: 477–484.
Jiang, W., B. Xia, J. Huang, and Z. Liu (2013) Characterization of a serine hydroxymethyltransferase for L-serine enzymatic production from Pseudomonas plecoglossicida. World J. Microb. Biot. 29: 2067–2076.
Peters-Wendisch, P., M. Stolz, H. Etterich, N. Kennerknecht, H. Sahm, and L. Eggeling (2005) Metabolic engineering of Corynebacterium glutamicum for L-serine production. Appl. Environ. Microbiol. 71: 7139–7144.
Stolz, M., P. Peters-Wendisch, H. Etterich, T. Gerharz, R. Faurie, H. Sahm, and L. Eggeling (2007) Reduced folate supply as a key to enhanced L-serine production by Corynebacterium glutamicum. Appl. Environ. Microbiol. 73: 750–755.
Zhu, Q., X. Zhang, Y. Luo, W. Guo, G. Xu, J. Shi, and Z. Xu (2015) L-Serine overproduction with minimization of by-product synthesis by engineered Corynebacterium glutamicum. Appl. Microbiol. Biot. 99: 1665–1673.
Gu, P., F. Yang, T. Su, F. Li, Y. Li, and Q. Qi (2014) Construction of an L-serine producing Escherichia coli via metabolic engineering. J. Ind. Microbiol. Biotechnol. 41: 1443–1450.
Mundhada, H., K. Schneider, H. B. Christensen, and A. T. Nielsen (2016) Engineering of high yield production of L?serine in Escherichia coli. Biotechnol. Bioeng. 113: 807–816.
Mundhada, H., J. M. Seoane, K. Schneider, A. Koza, H. B. Christensen, T. Klein, and A. T. Nielsen (2017) Increased production of L-serine in Escherichia coli through adaptive laboratory evolution. Metab. Eng. 39: 141–150.
Lin, Z., Z. Xu, Y. Li, Z. Wang, T. Chen, and X. Zhao (2014) Metabolic engineering of Escherichia coli for the production of riboflavin. Microb. Cell. Fact. 13: 104.
Kuhlman, T. E. and E. C. Cox (2010) Site-specific chromosomal integration of large synthetic constructs. Nucleic Acids Res. 38: e92.
Zhang, Y., Z. Lin, Q. Liu, Y. Li, Z. Wang, H. Ma, and X. Zhao (2014) Engineering of Serine-Deamination pathway, Entner-Doudoroff pathway and pyruvate dehydrogenase complex to improve poly(3-hydroxybutyrate) production in Escherichia coli. Microb. Cell. Fact. 13: 172.
Li, Y., G. K. Chen, X. W. Tong, H. T. Zhang, X. G. Liu, Y. H. Liu, and F. P. Lu (2012) Construction of Escherichia coli strains producing L-serine from glucose. Biotechnol. Lett. 34: 1525–1530.
Pizer, L. I. and M. L. Potochny (1964) Nutritional and regulatory aspects of serine metabolism in Escherichia coli. J. Bacteriol. 88: 611–619.
Zhang, X. and E. Newman (2008) Deficiency in l-serine deaminase results in abnormal growth and cell division of Escherichia coli K-12. Mol. Microbiol. 69: 870–881.
Plamann, M. D., W. D. Rapp, and G. V. Stauffer (1983) Escherichia coli K12 mutants defective in the glycine cleavage enzyme system. Mol. Genet. Genom. 192: 15–20.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, Y., Kang, P., Liu, S. et al. glyA gene knock-out in Escherichia coli enhances L-serine production without glycine addition. Biotechnol Bioproc E 22, 390–396 (2017). https://doi.org/10.1007/s12257-017-0084-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-017-0084-5