Abstract
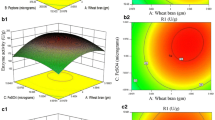
The optimal conditions for production of carboxymethylcellulase (CMCase) of Bacillus amyloliquefaciens DL-3 by a recombinant Escherichia coli JM109/DL-3 were established at a flask scale using the response surface method (RSM). The optimal conditions of rice bran, tryptone, and initial pH of the medium for cell growth extracted by Design Expert Software were 66.1 g/L, 6.2 g/L, and 7.2, respectively, whereas those for production of CMCase were 58.0 g/L, 5.0 g/L, and 7.1. The analysis of variance (ANOVA) of results from central composite design (CCD) indicated that significant factor (“probe > F” less than 0.0500) for cell growth was rice bran, whereas those for production of CMCase were rice bran and initial pH of the medium. The optimal temperatures for cell growth and the production of CMCase by E. coli JM109/DL-3 were found to be 37°C. The optimal agitation speed and aeration rate of 7 L bioreactors for cell growth were 498 rpm and 1.4 vvm, whereas those for production of CMCase were 395 rpm and 1.1 vvm. The ANOVA of results indicated that the aeration rate was more significant factor (“probe > F” less than 0.0001) than the agitation speed for cell growth and production of CMCase. The optimal inner pressure for cell growth was 0.08 MPa, whereas that for the production of CMCase was 0.06 MPa. The maximal production of CMCase by E. coli JM109/DL-3 under optimized conditions was 871.0 U/mL, which was 3.0 times higher than the initial production of CMCase before optimization.
Similar content being viewed by others
References
Han, M. H., Y. L. Kim, Y. R. Kim, B. W. Chun, and G. W. Choi (2011) Bioethanol production from optimized pretreatment of cassava stem. Kor. J. Chem. Eng. 28: 119–125.
Blumer-Schuette, S. E., I. Kataeva, J. Westpheling, M. W. W. Adams, and R. M. Kelly (2008) Extremely thermophilic microorganisms for biomass conversion: Status and prospects. Curr. Opin. Biotechnol. 19: 210–217.
Li, H. X., L, Yang, Y. J. Kim, and S. J. Kim (2011) Continuous ethanol production by the synchronous saccharification and fermentation using food wastes. Kor. J. Chem. Eng. 28: 1085–1089.
Wei, G. Y., W. Gao, I. H. Jin, S. Y. Yoo, J. H. Lee, C. H. Chung, and J. W. Lee (2009) Pretreatment and saccharification of rice hulls for the production of fermentable sugars. Biotechnol. Bioproc. Eng. 14: 828–834.
Sukumaran, R. K., R. R. Singhania, G. M. Mathew, and A. Pandey (2009) Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew. Energy 34: 421–424.
Golias, H., G. J. Dumsday, G. A. Stanley, and N. B. Pamment (2000) Characteristics of cellulase preparation affecting the simultaneous saccharification and fermentation of cellulose to ethanol. Biotechnol. Lett. 26: 617–621.
Howard, R. L., E. Abotsi, E. L. Jansen von Rensburg, and S. Howard (2003) Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2: 602–619.
Yu, X. B., J. H. Nam, H. S. Yun, and T. M. Koo (1998) Optimization of cellulose production in batch fermentation by Trichoderma reesei. Biotechnol. Bioproc. Eng. 3: 44–47.
Takashima, S., H. Iikura, A. Nakamura, M. Hidaka, H. Masaki, and T. Uozumi (1998) Overproduction of recombinant Trichoderma reesei cellulases by Aspergillus oryzae and their enzymatic properties. J. Biotechnol. 65: 163–171.
Roboson, L. M. and G. H. Chambliss (1989) Cellulases of bacterial origin. Enz. Microb. Technol. 11: 626–644.
Shima, S., J. Kato, Y. Igarashi, and T. Kodama (1989) Cloning and expression of a Clostridium cellobioparum cellulase gene and its excretion from Escherichia coli JM109. J. Fermen. Bioeng. 68: 75–78.
Lee, Y. J., B. K. Kim, B. H. Lee, K. I. Jo, N. K. Lee, C. H. Chung, Y. C. Lee, and J. W. Lee (2008) Purification and characterization of cellulase produced by Bacillus amyloliquefaciens DL-3 utilizing rice hull. Bioresour. Technol. 99: 378–386.
Cho, H. H., Y. B. Kim, and E. K. Kim (2009) Optimization of culture media for Bacillus species by statistical experiment design. Kor. J. Chem. Eng. 26: 754–759.
Jo, K. I., Y. J. Lee, B. K. Kim, B. H. Lee, C. H., Chung, S. W. Nam, S. K. Kim, and J. W. Lee (2008) Pilot-scale production of carboxymethylcellulase from rice hull by Bacillus amyloliquefaciens DL-3. Biotechnol. Bioproc. Eng. 13: 182–188.
Kim, K. C., S. S. Yoo, Y. A. OH, and S. J. Kim (2003) Isolation and characteristics of Trichoderma harzianum FJ1 producing cellulases and xlyanase. J. Microbiol. Biotechnol. 13: 1–8.
Jecu, L. (2000) Solid state fermentation of agricultural wastes for endogulcanse production. Ind. Crops Prod. 11: 1–5.
Lee, S. M. and Y. M. Koo (2001) Pilot-scale production of cellulose using Trichoderma reesei Rut C-30 in fed-batch mode. J. Microbiol. Biotechnol. 11: 229–233.
Tao, S., L. Beihui, L. Zuohu, and L. Deming (1999) Effects of air pressure amplitude on productivity by Trichoderma viride SL-1 in periodic pressure solid state fermenter. Proc. Biochem. 34: 25–29.
Emtiazi, G. and I. Nahvi (2000) Multi-enzyme production by Cellulomonas sp. grown on wheat straw. Biomass Bioenergy 19: 31–37.
Kang, S. W., Y. S. Park, J. S. Lee, S. I. Hong, and S. W. Kim (2004) Production of cellulase and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour. Technol. 91: 153–156.
Lee, B. H., B. K. Kim, Y. J. Lee, C. H. Chung, and J. W. Lee (2010) Industrial scale of optimization for the production of carboxymethylcellulase from rice bran by a marine bacterium, Bacillus subtilis subsp. subtilis A-53. Enz. Microb. Technol. 46: 38–42.
Kim, B. K., B. H. Lee, Y. J. Lee, I. H. Jin, C. H. Chung, and J. W. Lee (2009) Purification and characterization of carboxymethylcellulase isolated from a marine bacterium, Bacillus subtilis subsp. subtilis A-53. Enz. Microb. Technol. 44: 411–416.
Yi, J. C., J. C. Sandra, A. B. John, and T. C. Shu (1999) Production and distribution of endoglucanase, cellobiohydrolase, and β-glucosidase components of the cellulolytic system of Volvariella volvacea, the edible straw mushroom. Appl. Environ. Microbiol. 65: 553–559.
Khuri, A. I. and J. A. Cornell (1987) Response surfaces: Design and analysis. Marcel Dekker, NY, USA.
Krishna, C. (1999) Production of bacterial cellulases by a solid state bioprocessing of banana wastes. Bioresour. Technol. 69: 231–239.
Kalogeris, E., P. Christakopoulos, P. Katapodis, A. Alexious, S. Vlachou, D. Kekos, and B. J. Macris (2003) Production and characterization of cellulolytic enzymes from the thermophilic fungus Thermoascus aurantiacus under solid state cultivation of agricultural waste. Proc. Biochem. 38: 1099–1104.
Rajoka, M. I. and K. A. Malik (1997) Cellulase production by Cellulomonas biazotea cultured in media containing different cellulosic substrates. Bioresour. Technol. 59: 21–27.
Malinowska, E., W. Krzyczkowski, G. Lapienis, and F. Herold (2009) Improved simultaneous production of mycelial biomass and polysaccharides by submerged culture of Hericium erinaceum: Optimization using a central composite rotatable design (CCRD). J. Ind. Microbiol. Biotechnol. 36: 1513–1527.
Giavasis, I., L. M. Harvey, and B. McNeil (2006) The effect of agitation and aeration on the synthesis and molecular weight of gellan in batch cultures of Sphingmonas paucimobilis. Enz. Microb. Technol. 38: 101–108.
Elibol, M. and D. Ozer (2000) Influence of oxygen transfer on lipase production by Rhizopus arrhizus. Proc. Biochem. 36: 325–329.
Thiry, M. and D. Cinogolani (2002) Optimizing scale-up fermentation process. Trend. Biotechnol. 20: 103–105.
Junker, B. H. (2004) Scale-up methodologies for Escherichia coli and yeast fermentation process. J. Biosci. Bioeng. 97: 347–364.
Seo, H. P., C. H. Chung, S. K. Kim, R. A. Gross, D. L. Kaplan, and J. W. Lee (2004) Mass production of pullulan with optimized concentrations of carbon and nitrogen sources by Aureobaisdium pullulans HP-2001 in a 100 L bioreactor with the inner pressure. J. Microbiol. Biotechnol. 14: 237–242.
Charoenrat, T., M. Ketudat-Cairns, M. Jahic, A. Veide, and S. Enfors (2006) Increased total air pressure versus oxygen limitation for enhanced oxygen transfer and product formation in a Pichia pastoris recombinant protein process. Biochem. Eng. J. 30: 205–211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, YJ., Kim, HJ., Gao, W. et al. Statistical optimization for production of carboxymethylcellulase of Bacillus amyloliquefaciens DL-3 by a recombinant Escherichia coli JM109/DL-3 from rice bran using response surface method. Biotechnol Bioproc E 17, 227–235 (2012). https://doi.org/10.1007/s12257-011-0258-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-011-0258-5