Abstract
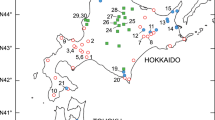
The Pilosella alpicola group includes four species (P. alpicola s.str., P. ullepitschii, P. rhodopea and P. serbica) with allopatric distributions (Alps, Balkans, Carpathians) and contrasting cytotype patterns (diploid, diploid-polyploid and polyploid species). Whereas diploid taxa (P. ullepitschii and P. serbica) reproduce sexually, the mode of reproduction of polyploid cytotypes reflects their origin: autopolyploids of P. rhodopea reproduce sexually, while allopolyploid cytotypes of P. alpicola s.str. apomictically. We used allozymes to elucidate overall genetic variation within the group and to test their utility for taxon discrimination, assessment of polyploid origin and possible correlations with breeding systems. Variation of five allozyme systems encoded by eight polymorphic loci and 29 alleles was studied in 20 populations and 298 plants representing all taxa. Allozymes were proved to be only of limited usefulness for the taxonomic classification within the P. alpicola group. The Western Carpathian populations of P. ullepitschii formed the only genetically well-differentiated group. The same allele suite shared by all cytotypes of P. rhodopea and presence of both balanced and unbalanced heterozygotes in tetraploids was consistent with autopolyploid origins of polyploids and provided further evidence for a primary contact zone. An isolated relic population of P. rhodopea from the Southern Carpathians exhibited lowered values of genetic diversity when compared to the core area. Pronounced fixed heterozygosity was found in P. alpicola s.str., supporting its allopolyploid origin. In accordance with assumptions, genotypic variability was significantly higher in sexually reproducing diploid and diploid-polyploid taxa than in apomictic P. alpicola s.str.





Similar content being viewed by others
References
Arft AM, Ranker TA (1998) Allopolyploid origin and population genetics of the rare orchid Spiranthes diluvialis. Amer J Bot 85:110–122
Bayer RJ (1991) Allozymic and morphological variation in Antennaria (Asteraceae: Inuleae) from the low arctic of Northwestern North America. Syst Bot 16:492–506
Bayer RJ, Crawford DJ (1986) Allozyme divergence among five diploid species of Antennaria (Asteraceae, Inuleae) and their allopolyploid derivatives. Amer J Bot 73:287–296
Bräutigam S, Greuter W (2007) A new treatment of Pilosella for the Euro-Mediterranean flora [Notulae ad floram euro-mediterraneam pertinentes No. 24]. Willdenowia 37:123–137
Brochmann C, Soltis DE, Soltis PS (1992) Electrophoretic relationships and phylogeny of Nordic polyploids in Draba (Brassicaceae). Pl Syst Evol 182:35–70
Bruun HH, Scheepens JF, Tyler T (2007) An allozyme study of sexual and vegetative regeneration in Hieracium pilosella L. Canad J Bot 85:10–15
Chessel D, Dufour AB, Thioulouse J (2004) The ade4 package-I- One-table methods. R News 4:5–10
Chrtek J Jr, Plačková I (2005) Genetic variation in Hieracium alpinum (Asteraceae) in the Krkonoše Mts (West Sudeten Mts, Czech Republic). Biologia, Bratislava 60:387–391
Crawford DJ, Archibald JK, Santos-Guerra A, Mort ME (2006) Allozyme diversity within and divergence among species of Tolpis (Asteraceae-Lactuceae) in the Canary Islands: Systematic, evolutionary, and biogeographical implications. Amer J Bot 93:656–664
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Eckert CG, Barrett SCH (1993) Clonal reproduction and patterns of genotypic diversity in Decodon verticillatus (Lythraceae). Amer J Bot 80:1175–1182
Ellstrand NC, Roose ML (1987) Patterns of genotypic diversity in clonal plant species. Amer J Bot 74:121–131
Fehrer J, Šimek R, Krahulcová A, Krahulec F, Chrtek J Jr, Bräutigam E, Bräutigam S (2005) Evolution, hybridisation, and clonal distribution of apo- and amphimictic species of Hieracium subgen. Pilosella (Asteraceae: Lactuceae) in a Central European mountain range. In Bakker FT, Chatrou LW, Gravendeel B, Pelser P (eds) Plant species-level systematics: new perspectives on pattern & process. Regnum Veg 143:175–201
Fehrer J, Gemeinholzer B, Chrtek J Jr, Bräutigam S (2007) Incongruent plastid and nuclear DNA phylogenies reveal ancient intergeneric hybridization in Pilosella hawkweeds (Hieracium, Cichorieae, Asteraceae). Molec Phylogen Evol 42:347–361
Gauthier P, Lumaret R, Bédécarrats A (1998) Genetic variation and gene flow in Alpine diploid and tetraploid populations of Lotus (L. alpinus (D.C.) Schleicher/L. corniculatus L.). I. Insights from morphological and allozyme markers. Heredity 80:683–693
Gottlieb LD (1981) Electrophoretic evidence and plant populations. Progr Phytochem 7:1–46
Gottlieb LD (1984) Isozyme evidence and problem solving in plant systematics. In Grant WF (ed) Plant biosystematics. Academic Press, London, pp 343–357
Goudet J (1995) FSTAT: a computer program to calculate F-statistics (version 2.9.3). J Heredity 86:485–486
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In Brown AHD, Clegg MT, Kahler AL, Wier BS (eds) Plant population genetics. Sinauer, Sunderland, Massachusetts, pp 43–63
Hardy OJ, Vekemans X (2001) Patterns of allozyme variation in diploid and tetraploid Centaurea jacea at different spatial scales. Evolution 55:943–954
Hardy OJ, Vanderhoeven S, De Loose M, Meerts P (2000) Ecological, morphological and allozymic differentiation between diploid and tetraploid knapweeds (Centaurea jacea) from a contact zone in the Belgian Ardennes. New Phytol 146:281–290
Hoebee SE, Menn C, Rotach P, Finkeldey R, Holderegger R (2006) Spatial genetic structure of Sorbus torminalis: The extent of clonal reproduction in natural stands of a rare tree species with a scattered distribution. Forest Ecol Managem 226:1–8
Hörandl E (2004) Comparative analysis of genetic divergence among sexual ancestors of apomictic complexes using isozyme data. Int J Pl Sci 165:615–622
Hörandl E, Greilhuber J (2002) Diploid and autotetraploid sexuals and their relationships to apomicts in the Ranunculus cassubicus group: insights from DNA content and isozyme variation. Pl Syst Evol 234:85–100
Hörandl E, Greilhuber J, Dobeš C (2000) Isozyme variation within the apomictic Ranunculus auricomus complex: evidence for a sexual progenitor species in southeastern Austria. Pl Biol 2:1–10
Hörandl E, Jakubowsky G, Dobeš C (2001) Isozyme and morphological diversity within apomictic and sexual taxa of the Ranunculus auricomus complex. Pl Syst Evol 226:165–185
Hughes J and Richards AJ (1988) The genetic structure of populations of sexual and asexual Taraxacum (dandelions). Heredity 60:161–171
Husband BC, Sabara HA (2004) Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium (Onagraceae). New Phytol 161:703–713
Jombart T. (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Kang SS, Chung MG (2000) High levels of allozyme variations and low allozyme divergence within and among species of Hemerocallis (Liliaceae). Amer J Bot 87:1634–1646
Karl SA, Avise JC (1992) Balancing selection at allozyme loci in oysters: implications from nuclear RFLPs. Science 256:100–102
Karl I, Schmitt T, Fischer K (2009) Genetic differentiation between alpine and lowland populations of a butterfly is related to PGI enzyme genotype. Ecography 32:488–496
Karron JD (1991) Patterns of genetic variation and breeding systems in rare plant species. In Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, Oxford, pp 87–98
Kashin AS, Anfalov VE, Demochko YuA (2005) Studying allozyme variation in sexual and apomictic Taraxacum and Pilosella (Asteraceae) populations. Russ J Genet 41:144–154
Kato T (1987) Hybridization between Dianthus superbus var. longicalycinus and D. shinanensis evidenced by resolvable esterase isozymes from herbarium specimens. Ann Tsukuba Bot Gard 6:9–18
Kimura M (1968) Evolutionary rate at the molecular level. Nature 217:624–626
Koehn RK, Hilbish TJ (1987) The adaptative importance of genetic variation. Amer Sci 75:134–141
Kolář F, Štech M, Trávníček P, Rauchová J, Urfus T, Vít P, Kubešová M, Suda J (2009) Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Ann Bot (Oxford) 103:963–974
Koltunow AM, Johnson SD, Bicknell RA (1998) Sexual and apomictic development in Hieracium. Sex Pl Reprod 11:213–230
Krahulcová A, Krahulec F, Chapman HM (2000) Variation in Hieracium subgen. Pilosella (Asteraceae): What do we know about its sources? Folia Geobot 35:319–338
Krahulcová A, Papoušková S, Krahulec F (2004) Reproduction mode in the allopolyploid facultatively apomictic hawkweed Hieracium rubrum (Asteraceae, H. subgen. Pilosella). Hereditas 141:19–30
Krahulcová A, Rotreklová O, Krahulec F, Rosenbaumová R, Plačková I (2009a) Enriching ploidy level diversity: the role of apomictic and sexual biotypes of Hieracium subgen. Pilosella (Asteraceae) that coexist in polyploid populations. Folia Geobot 44:281–306
Krahulcová A, Vladimirov V, Krahulec F, Bräutigam S (2009b) The agamic complex of Pilosella (Asteraceae) in Bulgaria and SW Romania: variation in ploidy level and breeding systems. Phytol Balcan 15:377–384
Krahulec F, Krahulcová A, Fehrer J, Bräutigam S, Plačková I, Chrtek J Jr (2004) The Sudetic group of Hieracium subgen. Pilosella from the Krkonoše Mts: a synthetic view. Preslia 76:223–243
Liston A, Wilson BL, Doescher PS, Robinson WA, Harris N, Svejcar T (2003) Genetic evidence for sexual and clonal reproduction in a 59-year old population of Festuca idahoensis (Poaceae). Oecologia 137:216–225
López-Pujol J, Bosch M, Simon J, Blanche C (2004) Allozyme diversity in the tetraploid endemic Thymus loscossi (Lamiaceae). Ann Bot (Oxford) 93:323–332
Mahy G, Bruederle LP, Connors B, Van Hofwegen M, Vorsa N (2000) Allozyme evidence for genetic autopolyploidy and high genetic diversity in tetraploid cranberry, Vaccinium oxycoccos (Ericaceae). Amer J Bot 87:1882–1889
Mráz P, Chrtek J Jr, Fehrer J, Plačková I (2005) Rare recent natural hybridization in Hieracium s.str. – evidence from morphology, allozymes and chloroplast DNA. Pl Syst Evol 255:177–192
Mráz P, Gaudeul M, Rioux D, Gielly L, Choler P, Taberlet P, IntraBioDiv Consortium (2007) Genetic structure of Hypochaeris uniflora (Asteraceae) suggests vicariance in the Carpathians and rapid post-glacial colonisation of the Alps from an Eastern Alpine refugium. J Biogeogr 34:2100–2114
Nägeli C, Peter A (1885) Die Hieracien Mittel-Europas. Monographishe Bearbeitung der Piloselloiden mit besonderer Berücksichtigung der mitteleuropäischen Sippen I. R. Oldenburg, München.
Nei M (1972) Genetic distances between populations. Amer Naturalist 106:283–292
Noyes RD, Soltis DE (1996) Genotypic variation in agamospermous Erigeron compositus (Asteraceae). Amer J Bot 83:1292–1303
Nyárády EI (1955) Vegetaţia muntelui Cozia şi cîteva plante noi pentru flora Olteniei, Moldovei şi Transilvaniei (Vegetation of the Cozia Mts and some new plants for the flora of Oltenia, Moldova and Transylvania). Bul Şti Secţ Sţi Biol, Agron, Geol Geogr 7:209–246
Obbard DJ, Harris SA, Pannell JR (2006) Simple allelic-phenotype diversity and differentiation statistics for allopolyploids. Heredity 97:296–303
Peckert T, Chrtek J Jr (2006) Mating interactions among coexisting diploid, triploid and tetraploid cytotypes of Hieracium echioides (Asteraceae). Folia Geobot 41:323–334
Peckert T, Chrtek J Jr, Plačková I (2005) Genetic variation in agamospermous populations of Hieracium echioides in southern Slovakia and northern Hungary (Danube Basin). Preslia 77:307–315
Pielou EC (1969) An introduction to mathematical ecology. John Wiley, New York.
Pogan E, Wcisło H (1995) Embryological analysis of Hieracium pilosella L. from Poland. Acta Biol Cracov, Ser Bot 37:53–61
Popescu A, Sanda V, Roman N, Şerbănescu GH, Doniţă N (1970) Investigations on the Olt gorge flora. Rev Roum Biol 15:259–269
Prentice HC, Lönn M, Lager H, Rosén E, van der Maarel E (2000) Changes in allozyme frequencies in Festuca ovina populations after a nine-year nutrient/water experiment. J Ecol 88:331–347
Puşcaş M, Choler P, Tribsch A, Gielly L, Rioux D, Gaudeul M, Taberlet P (2008) Post-glacial history of the dominant alpine sedge Carex curvula in the European Alpine System inferred from nuclear and chloroplast markers. Molec Ecol 17:2417–2429
Ramsey J, Schemske DW (2002) Neopolyploidy in flowering plants. Annual Rev Ecol Syst 33:589–639
Reed JM, Kryštufek B, Eastwood WJ (2004) The physical geography of the Balkans and nomenclature of place names. In Griffths HI, Kryštufek B, Reed JM (eds) Balkan biodiversity pattern and process in the European hotspot. Kluwer Academic Publishers, Dordrecht, pp 9–22
Ronikier M, Cieślak E, Korbecka G (2008) High genetic differentiation in the alpine plant Campanula alpina Jacq. (Campanulaceae): evidence for glacial survival in several Carpathian regions and long isolation between the Carpathians and the Eastern Alps. Molec Ecol 17:1763–1775
Roose ML, Gottlieb LD (1976) Genetic and biochemical consequences of polyploidy in Tragopogon. Evolution 30:818–830
Rosquist G, Prentice HC (2002) Genetic variation in Scandinavian Anthericum liliago (Anthericaceae): allopolyploidy, hybridization and immigration history. Pl Syst Evol 236:55–72
Rotreklová O, Krahulcová A, Vaňková D, Peckert T, Mráz P (2002) Chromosome numbers and breeding systems in some species of Hieracium subgen. Pilosella from Central Europe. Preslia 74:27–44
Ruiz E, Crawford DJ, Stuessy TF, Gonzalez F, Samuel R, Becerra J, Silva M (2004) Phylogenetic relationships and genetic divergence among endemic species of Berberis, Gunnera, Myrceugenia and Sophora of the Juan Fernandez Islands (Chile) and their continental progenitors based on isozymes and nrITS sequences. Taxon 53:321–332
Samuel R, Pinsker W, Ehrendorfer F (1990) Allozyme polymorphism in diploid and polyploid populations of Galium. Heredity 65:369–378
Schlaepfer DR, Edwards PJ, Semple JC, Billeter R (2008) Cytogeography of Solidago gigantea (Asteraceae) and its invasive ploidy level. J Biogeogr 35: 2119–2127
Schmitt T (2009) Biogeographical and evolutionary importance of the European high mountain systems. Frontiers Zool 6:9
Schuhwerk F (1996) Published chromosome-counts in Hieracium. Available at: http://www.botanischestaatssammlung.de/projects/chrzlit.html
Šingliarová B, Hodálová I, Mráz P (2011) A biosystematic study of the diploid-polyploid Pilosella alpicola group with variation in breeding system: patterns and processes. Taxon 60:450–470
Šingliarová B, Mráz P (2009) A taxonomic revision of the Pilosella alpicola group (Asteraceae, Lactuceae) in the Carpathians. Preslia 81:23–41
Šingliarová B, Chrtek J Jr, Mráz P (2008) Loss of genetic diversity in isolated populations of an alpine endemic Pilosella alpicola subsp. ullepitschii: effect of long-term vicariance or long-distance dispersal? Pl Syst Evol 275:181–191
Sipes SD, Wolf PG (1997) Clonal structure and patterns of allozyme diversity in the rare endemic Cycladenia humilis var. jonesii (Apocynaceae). Amer J Bot 84:401–409
Soltis DE, Rieseberg LH (1986) Autopolyploidy in Tolmiea menziesii (Saxifragaceae): genetic insights from enzyme electrophoresis. Amer J Bot 73:310–318
Soltis DE, Soltis PS (1989) Genetic consequences of autopolyploidy in Tolmiea (Saxifragaceae). Evolution 43:586–594
Soltis DE, Soltis PS (1990) Chloroplast DNA and nuclear rDNA variation: insights into autopolyploid and allopolyploid evolution. In Kawano S (ed) Biological approaches and evolutionary trends in plants. Academic Press, San Diego, pp 97–117
Soltis DE, Soltis PS (1993) Molecular data and the dynamic nature of polyploidy. Crit Rev Pl Sci 12:243–273
Soltis PS, Soltis DE (2000) The role of genetic and genomic attributes in the succes of polyploids. Proc Natl Acad Sci USA 97:7051–7057
Stebbins GL (1980) Polyploidy in plants: unsolved problems and prospects. In Lewis WH (ed) Polyploidy, biological relevance. Plenum Press, New York and London, pp 495–520
Štorchová H, Chrtek J Jr, Bartish IV, Tetera M, Kirschner J, Štěpánek J (2002) Genetic variation in agamospermous taxa of Hieracium sect. Alpina (Compositae) in the Tatry Mts (Slovakia). Pl Syst Evol 235:1–17
Stuessy TF, Weiss-Schneeweiss H, Keil DJ (2004) Diploid and polyploid cytotype distribution in Melampodium cinereum and M. leucanthum (Asteraceae, Heliantheae) Amer J Bot 91:889–898
Sun M, Wong KC (2001) Genetic structure of three orchid species with contrasting breeding systems using RAPD and allozyme markers. Amer J Bot 88:2180–2189
Sydes MA, Peakall R (1998) Extensive clonality in the endangered shrub Haloragodendron lucasii (Haloragaceae) revealed by allozymes and RAPDs. Molec Ecol 7:87–93
Těšitel J, Malinová T, Štech M, Herbstová M (2009) Variation in the Melampyrum sylvaticum group in the Carpathian and Hercynian region: two lineages with different evolutionary histories. Preslia 81:1–22
Thompson JD, Lumaret R (1992) The evolutionary dynamics of polyploid plants: origins, establishment and persistence. Trends Ecol Evol 7:302–307
Trávníček P, Dočkalová Z, Rosenbaumová R, Kubátová D, Szeląg Z, Chrtek J (2011) Bridging global and microregional scales: ploidy distribution in Pilosella echioides (Asteraceae) in central Europe. Ann Bot (Oxford) 107:443–454
Tyler T (2001) Forslag till ny taxonomisk indelning av stangfibblorna (Pilosella) i Norden (The genus Pilosella in the Nordic countries). Svensk Bot Tidskr 95:39–67
Tyler T (2005) Patterns of allozyme variation in Nordic Pilosella. Pl Syst Evol 250:133–145
Varga ZS, Schmitt T (2008) Types of oreal and oreotundral disjunctions in the western Palearctic. Biol J Linn Soc 93:415–430
Weeden NF, Wendel JF (1989) Genetics of plant isozymes. In Soltis P, Soltis D (eds) Isozymes in plant biology. Dioscorides Press, Portland, pp 46–72
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Weiss H, Dobeš C, Schneeweiss GM, Greimler J (2002) Occurrence of tetraploid and hexaploid cytotypes between and within populations in Dianthus sect. Plumaria (Caryophyllaceae). New Phytol 156:85–94
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Yahara T (1990) Evolution of agamospermous races in Boehmeria and Eupatorium. Pl Spec Biol 5:183–196
Yeh FC, Yang R-C, Boyle T (1999) POPGENE version 1.32. Microsoft Window-based freeware for population genetic analysis. Available at: http://www.ualberta.ca/~fyeh/
Zahn KH (1922–1930) Hieracium. In Ascherson P, Graebner P (eds) Synopsis der mitteleuropäischen Flora 12 (1), Gebrüder Borntraeger, Leipzig
Acknowledgements
We would like to thank Róbert Lakoštík (Bratislava) for his valuable assistance in the field, Róbert Šuvada (Brzotín) for helping with the map preparation, Frederic Rooks (Praha) for language correction and two anonymous reviewers for helpful suggestions. This study was financially supported by the Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences (VEGA 1/0028/08 to PM), the Academy of Sciences of the Czech Republic (AV0Z 60050516, to JC) and the Ministry of Education, Youth and Sports of the Czech Republic (0021620828, to JC).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Material 1
Allelic frequencies in eight polymorphic loci in all analyzed populations of the Pilosella alpicola group. Species-unique alleles are indicated in bold (DOC 132 kb)
Rights and permissions
About this article
Cite this article
Šingliarová, B., Chrtek, J., Plačková, I. et al. Allozyme Variation in Diploid, Polyploid and Mixed-Ploidy Populations of the Pilosella alpicola Group (Asteraceae): Relation to Morphology, Origin of Polyploids and Breeding System. Folia Geobot 46, 387–410 (2011). https://doi.org/10.1007/s12224-011-9102-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-011-9102-0