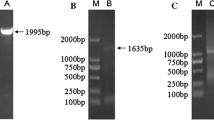
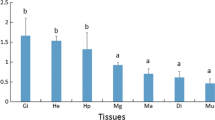
Abstract
Heat shock proteins (Hsps) are a class of highly conserved proteins produced in virtually all living organisms from bacteria to humans. Hsp60 and Hsp10, the most important mitochondrial chaperones, participate in environmental stress responses. In this study, the full-length complementary DNAs (cDNAs) of Hsp60 (PmHsp60) and Hsp10 (PmHsp10) were cloned from Penaeus monodon. Sequence analysis showed that PmHsp60 and PmHsp10 encoded polypeptides of 578 and 102 amino acids, respectively. The expression profiles of PmHsp60 and PmHsp10 were detected in the gills and hepatopancreas of the shrimps under pH challenge, osmotic stress, and heavy metal exposure, and results suggested that PmHsp60 and PmHsp10 were involved in the responses to these stimuli. ATPase and chaperone activity assay indicated that PmHsp60 could slow down protein denaturation and that Hsp60/Hsp10 may be combined to produce a chaperone complex with effective chaperone and ATPase activities. Overall, this study provides useful information to help further understand the functional mechanisms of the environmental stress responses of Hsp60 and Hsp10 in shrimp.













Similar content being viewed by others
References
Ahsanullah M, Negilski D, Mobley M (1981) Toxicity of zinc, cadmium and copper to the shrimp Callianassa australiensis III accumulation metals. Mar Biol 64:311–316
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Boettinger L, Oeljeklaus S, Guiard B, Rospert S, Warscheid B, Becker T (2015) The mitochondrial heat shock protein 70 (Hsp70) and Hsp10 cooperate in the formation of Hsp60 complexes. J Biol Chem 290:11611–11622. doi:10.1074/jbc.M115.642017
Burdon RH, Cutmore CM (1982) Human heat shock gene expression and the modulation of plasma membrane Na+, K+-ATPase activity. FEBS Lett 140:45–48
Calabrese A, MacInnes J, Nelson D, Miller J (1977) Survival and growth of bivalve larvae under heavy-metal stress. Mar Biol 41:179–184
Cappello F et al (2006) Hsp60 and Hsp10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease. Cancer 107:2417–2424. doi:10.1002/cncr.22265
Cappello F, Marino Gammazza A, Palumbo Piccionello A, Campanella C, Pace A, Conway de Macario E, Macario AJ (2014) Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opin Ther Targets 18:185–208. doi:10.1517/14728222.2014.856417
Cesar JR, Yang J (2007) Expression patterns of ubiquitin, heat shock protein 70, alpha-actin and beta-actin over the molt cycle in the abdominal muscle of marine shrimp Litopenaeus vannamei. Mol Reprod Dev 74:554–559. doi:10.1002/mrd.20605
Chatterjee M, Das P, Mazumder A, Nagdas SK, Sen PC (2009) Localization and expression of a 70 kDa protein in goat spermatozoa having Na+, K + -ATPase inhibitory and arylsulphatase A activities. Mol Cell Biochem 321:85–94. doi:10.1007/s11010-008-9922-2
Chen HC (1985) Water quality criteria for farming the grass shrimp, Penaeus monodon. In 1985. Aquaculture Department, Southeast Asian Fisheries Development Center, p 165
Chen JC, Lin CH (2001) Toxicity of copper sulfate for survival, growth, molting and feeding of juveniles of the tiger shrimp. Penaeus monodon Aquac 192:55–65. doi:10.1016/S0044-8486(00)00442-7
Chen X, Zhu YH, Cheng XY, Zhang Z, Xu S (2012) The Protection of Selenium against Cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules 17:14565–14572. doi:10.3390/molecules171214565
Clare DK, Saibil HR (2013) ATP-driven molecular chaperone machines. Biopolymers 99:846–859. doi:10.1002/bip.22361
Costa MP et al (2011) Molecular characterization of the Corynebacterium pseudotuberculosis hsp60-hsp10 operon, and evaluation of the immune response and protective efficacy induced by hsp60 DNA vaccination in mice. BMC Res Notes 4:243. doi:10.1186/1756-0500-4-243
Cui S et al (2011) A macrophage migration inhibitory factor like oxidoreductase from pearl oyster Pinctada fucata involved in innate immune responses. Fish Shellfish Immunol 31:173–181. doi:10.1016/j.fsi.2011.03.009
Cui Z, Liu Y, Luan W, Li Q, Wu D, Wang S (2010) Molecular cloning and characterization of a heat shock protein 70 gene in swimming crab (Portunus trituberculatus). Fish Shellfish Immunol 28:56–64. doi:10.1016/j.fsi.2009.09.018
Deane E, Woo N (2005) Evidence for disruption of Na+‐K+‐ATPase and hsp70 during vibriosis of sea bream, Sparus (=Rhabdosargus) sarba Forsskål. J Fish Dis 28:239–251. doi:10.1111/j.1365-2761.2005.00624.x
Dorts J, Silvestre F, Tu HT, Tyberghein AE, Phuong NT, Kestemont P (2009) Oxidative stress, protein carbonylation and heat shock proteins in the black tiger shrimp. Penaeus monodon, following exposure to endosulfan and deltamethrin. Environ Toxicol Pharmacol 28:302–310. doi:10.1016/j.etap.2009.05.006
Freeman BC, Michels A, Song J, Kampinga HH, Morimoto RI (2000) Analysis of molecular chaperone activities using in vitro and in vivo approaches. In: Stress Response, vol 2000. Springer, pp 393–419
Furriel RP, Masui DC, McNamara JC, Leone FA (2004) Modulation of gill Na+, K + -ATPase activity by ammonium ions: Putative coupling of nitrogen excretion and ion uptake in the freshwater shrimp Macrobrachium olfersii J Exp Zool A Comp. Exp Biol 301:63–74. doi:10.1002/jez.a.20008
Gething MJ, Sambrook J (1992) Protein folding in the cell. Nature 355:33–45
Ghosh JC, Dohi T, Kang BH, Altieri DC (2008) Hsp60 regulation of tumor cell apoptosis. J Biol Chem 283:5188–5194. doi:10.1074/jbc.M705904200
Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G (2011) Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol 31:960–968. doi:10.1161/ATVBAHA.110.217877
Guhathakurta H, Kaviraj A (2000) Heavy metal concentration in water, sediment, shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackish water ponds of Sunderban. India Mar Pollut Bull 40:914–920
Gupta RS, Ramachandra NB, Bowes T, Singh B (2008) Unusual cellular disposition of the mitochondrial molecular chaperones Hsp60, Hsp70 and Hsp10. In: Novartis Foundation symposium. Chichester; New York; John Wiley; 1999, p 59
Hansen JJ et al (2003) Genomic structure of the human mitochondrial chaperonin genes: HSP60 and HSP10 are localised head to head on chromosome 2 separated by a bidirectional promoter. Hum Genet 112:71–77. doi:10.1007/s00439-002-0837-9
Hayer Hartl M, Bracher A, Hartl FU (2015) The GroEL-GroES chaperonin machine: A nano-cage for protein folding. Trends Biochem Sci. doi:10.1016/j.tibs.2015.07.009
Hollander JM, Lin KM, Scott BT, Dillmann WH (2003) Overexpression of PHGPx and HSP60/10 protects against ischemia/reoxygenation injury free radical. Biol Med 35:742–751
Huang PY, Kang ST, Chen WY, Hsu TC, Lo CF, Liu KF, Chen LL (2008) Identification of the small heat shock protein, HSP21, of shrimp Penaeus monodon and the gene expression of HSP21 is inactivated after white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol 25:250–257. doi:10.1016/j.fsi.2008.06.002
Huang WJ, Leu JH, Tsau MT, Chen JC, Chen LL (2011) Differential expression of LvHSP60 in shrimp in response to environmental stress. Fish Shellfish Immunol 30:576–582. doi:10.1016/j.fsi.2010.12.001
Huesca M, Goodwin A, Bhagwansingh A, Hoffman P, Lingwood CA (1998) Characterization of an acidic-pH-inducible stress protein (hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori. Infect Immun 66:4061–4067
Jha R, Vardhan H, Bas S, Salhan S, Mittal A (2011) Chlamydia trachomatis heat shock proteins 60 and 10 induce apoptosis in endocervical epithelial cells. Inflamm Res 60:69–78. doi:10.1007/s00011-010-0237-x
Jiang S, Qiu L, Zhou F, Huang J, Guo Y, Yang K (2009) Molecular cloning and expression analysis of a heat shock protein (Hsp90) gene from black tiger shrimp (Penaeus monodon). Mol Biol Rep 36:127–134. doi:10.1007/s11033-007-9160-9
Jing J, Liu H, Chen H, Hu S, Xiao K, Ma X (2013) Acute effect of copper and cadmium exposure on the expression of heat shock protein 70 in the Cyprinidae fish Tanichthys albonubes. Chemosphere 91:1113–1122. doi:10.1016/j.chemosphere.2013.01.014
Johnson SM et al (2014) A biochemical screen for GroEL/GroES inhibitors. Bioorg Med Chem Lett 24:786–789. doi:10.1016/j.bmcl.2013.12.100
Jung MY, Lee YM (2013) Expression profiles of heat shock protein gene families in the monogonont rotifer Brachionus koreanus-exposed to copper and cadmium. Toxicol Environ Health Sci 4:235–242. doi:10.1007/s13530-012-0141-6
Kilstrup M, Jacobsen S, Hammer K, Vogensen FK (1997) Induction of heat shock proteins DnaK. GroEL, and GroES by salt stress in Lactococcus lactis. Appl Environ Microbiol 63:1826–1837
Knoflach M et al (2011) Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circ J 75:2491–2495. doi:10.1253/circj.CJ-11-0196
Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA (2000) Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol 164:13–17. doi:10.4049/jimmunol.164.1.13
Kozlova T, Perezgasga L, Reynaud E, Zurita M (1997) The Drosophila melanogaster homologue of the hsp60 gene is encoded by the essential locus l (1) 10Ac and is differentially expressed during fly development. Dev Genes Evol 207:253–263
Li J et al (2012) Cloning of a heat shock protein 90 (HSP90) gene and expression analysis in the ridgetail white prawn Exopalaemon carinicauda. Fish Shellfish Immunol 32:1191–1197. doi:10.1016/j.fsi.2012.03.008
Li Y et al (2011) Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem 286:31308–31319. doi:10.1074/jbc.M111.246124
Lima AG, McNamara JC, Terra WR (1997) Regulation of hemolymph osmolytes and gill Na+/K+-ATPase activities during acclimation to saline media in the freshwater shrimp Macrobrachium olfersii (Wiegmann, 1836)(Decapoda, Palaemonidae). J Exp Mar Biol Ecol 215:81–91
Lo WY, Liu KF, Liao IC, Song YL (2004) Cloning and molecular characterization of heat shock cognate 70 from tiger shrimp (Penaeus monodon). Cell Stress Chaperones 9:332. doi:10.1379/csc-47r.1
Ma D et al (2013) Oxidative damages and ultrastructural changes in the sperm of freshwater crab Sinopotamon henanense exposed to cadmium. Ecotoxicol Environ Saf 98:244–249. doi:10.1016/j.ecoenv.2013.08.004
Magen D et al (2008) Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am J Hum Genet 83:30–42. doi:10.1016/j.ajhg.2008.05.016
Magnoni R, Palmfeldt J, Hansen J, Christensen JH, Corydon TJ, Bross P (2014) The Hsp60 folding machinery is crucial for manganese superoxide dismutase folding and function. Free Radic Res 48:168–179. doi:10.3109/10715762.2013.858147
Martin J, Horwich AL, Hartl FU (1992) Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science 258:995–998
Masui DC et al (2005) Gill microsomal (Na+, K+)-ATPase from the blue crab Callinectes danae: interactions at cationic sites. Int J Biochem Cell Biol 37:2521–2535. doi:10.1016/j.biocel.2005.06.004
Mazzei V et al (2015) Metallothioneins and heat shock proteins 70 in Armadillidium vulgare (Isopoda, Oniscidea) exposed to cadmium and lead. Ecotoxicol Environ Saf 116:99–106. doi:10.1016/j.ecoenv.2015.03.007
Meinhardt A, Parvinen M, Bacher M, Aumüller G, Hakovirta H, Yagi A, Seitz J (1995) Expression of mitochondrial heat shock protein 60 in distinct cell types and defined stages of rat seminiferous epithelium. Biol Reprod 52:798–807
Meury J, Kohiyama M (1991) Role of heat shock protein DnaK in osmotic adaptation of Escherichia coli. J Bacteriol 173:4404–4410
Meyran J, Graf F (1986) Ultrahistochemical localization of Na+-K+ ATPase, Ca2+-ATPase and alkaline phosphatase activity in a calcium-transporting epithelium of a crustacean during moulting. Histochemistry 85:313–320
Mokhtar MB, Aris AZ, Munusamy V, Praveena SM (2009) Assessment level of heavy metals in Penaeus monodon and Oreochromis spp. in selected aquaculture ponds of high densities development area. Eur J Sci Res 30:348–360
Music E, Khan S, Khamis I, Heikkila JJ (2014) Accumulation of heme oxygenase-1 (HSP32) in Xenopus laevis A6 kidney epithelial cells treated with sodium arsenite, cadmium chloride or proteasomal inhibitors. Comp Biochem Physiol C Toxicol Pharmacol 166:75–87. doi:10.1016/j.cbpc.2014.07.007
Neuer A, Spandorfer S, Giraldo P, Dieterle S, Rosenwaks Z, Witkin S (2000) The role of heat shock proteins in reproduction. Hum Reprod Update 6:149–159. doi:10.1093/humupd/6.2.149
Nisemblat S, Parnas A, Yaniv O, Azem A, Frolow F (2014) Crystallization and structure determination of a symmetrical 'football' complex of the mammalian mitochondrial Hsp60-Hsp10 chaperonins. Acta Crystallogr Sect F: Struct Biol Cryst Commun 70:116–119. doi:10.1107/S2053230X1303389X
Paranko J, Seitz J, Meinhardt A (1996) Developmental expression of heat shock protein 60 (HSP60) in the rat testis and ovary. Differentiation 60:159–167
Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, Rodriguez-Iturbe B (2013) Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol 304:F289–F299. doi:10.1152/ajprenal.00517.2012
Qian Z, Liu X, Wang L, Wang X, Li Y, Xiang J, Wang P (2012) Gene expression profiles of four heat shock proteins in response to different acute stresses in shrimp. Litopenaeus vannamei. Comp Biochem Physiol C 156:211–220. doi:10.1016/j.cbpc.2012.06.001
Quintana FJ, Cohen IR (2011) The HSP60 immune system network. Trends Immunol 32:89–95. doi:10.1016/j.it.2010.11.001
Rafiee P et al (2006) Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am J Physiol Cell Physiol 291:C931–C945. doi:10.1152/ajpcell.00474.2005
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis 33:789–801. doi:10.1111/j.1365-2761.2010.01183.x
Saibil HR, Fenton WA, Clare DK, Horwich AL (2013) Structure and allostery of the chaperonin GroEL. J Mol Biol 425:1476–1487. doi:10.1016/j.jmb.2012.11.028
Saibil HR, Ranson NA (2002) The chaperonin folding machine. Trends Biochem Sci 27:627–632. doi:10.1016/S0968-0004(02)02211-9
Sanchez GI, Carucci DJ, Sacci J, Resau JH, Rogers WO, Kumar N, Hoffman SL (1999) Plasmodium yoelii: cloning and characterization of the gene encoding for the mitochondrial heat shock protein 60. Exp Parasitol 93:181–190
Seebaugh DR, Wallace WG, L’Amoreaux WJ, Stewart GM (2012) Carbon assimilation and digestive toxicity in naive grass shrimp (Palaemonetes pugio) exposed to dietary cadmium. Bull Environ Contam Toxicol 88:449–455. doi:10.1007/s00128-011-0493-7
Shekhar MS, Kiruthika J, Ponniah AG (2013) Identification and expression analysis of differentially expressed genes from shrimp (Penaeus monodon) in response to low salinity stress. Fish Shellfish Immunol 35:1957–1968. doi:10.1016/j.fsi.2013.09.038
Suttnar J, Dyr J, Hamšíková E, Novak J, Vonka V (1994) Procedure for refolding and purification of recombinant proteins from Escherichia coli inclusion bodies using a strong anion exchanger Journal of Chromatography B. Biomed Sci Appl 656:123–126
Tang D et al (2011) High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab 13:701–711. doi:10.1016/j.cmet.2011.04.008
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tsai YL, Chiang YR, Wu CF, Narberhaus F, Lai EM (2012) One out of four: HspL but no other small heat shock protein of Agrobacterium tumefaciens acts as efficient virulence-promoting VirB8 chaperone. PLoS One 7:e49685. doi:10.1371/journal.pone.0049685
Tsan M-F, Gao B (2004) Heat shock protein and innate immunity. Cell Mol Immunol 1:274–279. doi:10.1046/j.1365-2249.2002.01759.x
Tyagi NK, Fenton WA, Horwich AL (2009) GroEL/GroES cycling: ATP binds to an open ring before substrate protein favoring protein binding and production of the native state. Proc Natl Acad Sci U S A 106:20264–20269. doi:10.1073/pnas.0911556106
Uccelletti D et al (2005) The Golgi Ca2+-ATPase KlPmr1p function is required for oxidative stress response by controlling the expression of the heat-shock element HSP60 in Kluyveromyces lactis. Mol Biol Cell 16:4636–4647. doi:10.1091/mbc.E05-02-0138
Wang S, Wang Y, Zhang Z, Hong H, Zheng W (2001) Effects of Cu 2+, Zn 2+, Cd 2+ on the Phototaxic Response of Artemia Salina and Two Shrimp (Penaeus penicillatus and Penaeus monodon) Nauplius. J Xiamen Univ (Nat Sci)/Xiamen Daxue Xuebao 40:1336–1339. doi:10.3321/j.issn:0438–0479.2001.06.030
Wang WN et al (2009) Oxidative stress. Comp Biochem Physiol C 150:428–435. doi:10.1016/j.cbpc.2009.06.010
Welch WJ (1991) The role of heat-shock proteins as molecular chaperones. Curr Opin Cell Biol 3:1033–1038
Wong C, Cheung J, Chu K (1995) Effects of copper on survival, development and growth of Metapenaeus ensis larvae and postlarvae (Decapoda: Penaeidae). Mar Pollut Bull 31:416–419
Wu SM, Jong KJ, Lee YJ (2006) Relationships among metallothionein, cadmium accumulation, and cadmium tolerance in three species of fish. Bull Environ Contam Toxicol 76:595–600. doi:10.1007/s00128-006-0961-7
Xanthoudakis S et al (1999) Hsp60 accelerates the maturation of pro‐caspase‐3 by upstream activator proteases during apoptosis. EMBO J 18:2049–2056
Xu D, Sun L, Liu S, Zhang L, Ru X, Zhao Y, Yang H (2014) Molecular cloning of heat shock protein 10 (Hsp10) and 60 (Hsp60) cDNAs and their expression analysis under thermal stress in the sea cucumber Apostichopus japonicus. Comp Biochem Physiol B 171:49–57. doi:10.1016/j.cbpb.2014.03.009
Xu Q, Qin Y (2012) Molecular cloning of heat shock protein 60 (PtHSP60) from Portunus trituberculatus and its expression response to salinity stress. Cell Stress Chaperones 17:589–601. doi:10.1007/s12192-012-0334-6
Xu Z, Horwich AL, Sigler PB (1997) The crystal structure of the asymmetric GroEL-GroES-(ADP) 7 chaperonin complex. Nature 388:741–750
Zhou J, Wang WN, Wang AL, He WY, Zhou QT, Liu Y, Xu J (2009) Glutathione S-transferase in the white shrimp Litopenaeus vannamei: characterization and regulation under pH stress. Comp Biochem Physiol C 150:224–230. doi:10.1016/j.cbpc.2009.04.012
Acknowledgments
This work was supported by the Special Fund for Fisheries-Scientific Research (A201300B03), the Special Scientific Research Funds for Central Non-profit Institutes (2015YD05, 2014TS12), the Guangdong Provincial Science and Technology Program (2013B040402016, 2014A020208039) and the Guangdong Province Marine Fishery Science and Technology and Industrial Development of Special Scientific and Technological Research and Development Projects (A201501A11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, J., Fu, M., Zhao, C. et al. Characterization and function analysis of Hsp60 and Hsp10 under different acute stresses in black tiger shrimp, Penaeus monodon . Cell Stress and Chaperones 21, 295–312 (2016). https://doi.org/10.1007/s12192-015-0660-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0660-6