Abstract
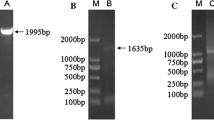
Heat shock protein 60 (HSP60) is a highly conserved and multi-functional molecular chaperone that plays an essential role in both cellular metabolism and stress response. Portunus trituberculatus is an important marine fishery and aquaculture species, and water salinity condition influenced its artificial propagations significantly. In order to investigate the function of P. trituberculatus HSP60 against osmotic stress, P. trituberculatus HSP60 gene was firstly cloned. The full-length cDNA of PtHSP60 contains 1,743 nucleotides encoding 577 amino acids with a calculated molecular weight of 61.25 kDa. Multiple alignments indicated that the deduced amino acid sequences of PtHSP60 shared a high level of identity with invertebrate and vertebrate HSP60 sequence including shrimp, fruit fly, zebrafish, and human. The expression profiles of PtHSP60 at mRNA and protein levels under salinity treatment were investigated by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analysis, respectively. It was found that the mRNA transcripts of PtHSP60 gene varied among different tissues under normal salinity conditions, and the antennal gland showed the highest expression level among the tissues tested. As for low salinity challenge, the mRNA expression of PtHSP60 gene was higher in the gill and appendicular muscle compared with other tissues, and gill and hypodermis represented the higher gene expressions during the hyperosmotic stress, which indicated that those tissues were salinity-sensitive tissues. In addition, salinity challenges significantly altered the expression of PtHSP60 at mRNA and protein level in a salinity- and time-dependent manner in P. trituberculatus gill tissue. The results indicate that PtHSP60 played important roles in mediating the salinity stress in P. trituberculatus.






Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bedwell DM, Strobel SA, Yun K, Jongeward GD, Emr SD (1989) Sequence and structural requirements of a mitochondrial protein import signal defined by saturation cassette mutagenesis. Mol Cell Biol 9:1014–1025
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bruce JL, Price BD, Coleman N, Calderwood SK (1993) Oxidant injury rapidly activates the heat shock transcription factor but fails to increase levels of heat shock proteins. Cancer Res 53:12–15
Chen Z, Christina CC-H, Zhang J, Cao L, Chen L, Zhou L, Jin Y, Ye H, Deng C, Dai Z, Xu Q, Hu P, Sun S, Shen Y, Chen L (2008) Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish. Proc Natl Acad Sci USA 105(35):12944–12949
Choresh O, Ron E, Loya Y (2001) The 60-kDa heat shock protein (HSP60) of the sea anemone Anemonia viridis: a potential early warning system for monitoring environmental changes. Mar Biotechnol 3:501–508
Choresh O, Loya Y, Muller WEG, Wiedenmann J, Azem A (2004) The mitochondrial 60-kDa heat shock protein in marine invertebrates: biochemical purification and molecular characterization. Cell Stress Chaperones 9:38–47
Clayton ME, Steinmann R, Fent K (2000) Different expression patterns of heat shock proteins hsp 60 and hsp 70 in zebra mussels (Dreissena polymorpha) exposed to copper and tributyltin. Aquat Toxicol 47:213–226
Dai AY (1977) Primary investigation on the fishery biology of the Portunus trituberculatus. Mar Fish 25:136–141 (in Chinese)
Dai AY, Yang SL, Song YZ (1986) Marine crabs in China Sea. Marine Publishing Company, Beijing, pp 194–196, in Chinese
Deane E, Kelly S, Luk J, Woo N (2002) Chronic salinity adaptation modulates hepatic heat shock protein and insulin-like growth factor I expression in black sea bream. Mar Biotechnol 4:193–205
Dong YW, Dong SL, Meng XL (2008) Effects of thermal and osmotic stress on growth, osmoregulation and Hsp70 in sea cucumber (Apostichopus japonicus Selenka). Aquaculture 276:179–186
Ellis RJ, van der Vies SM (1991) Molecular chaperones. Annu Rev Biochem 60:321–347
Emanuelsson O, von Heijne G, Schneider G (2001) Analysis and prediction of mitochondrial targeting peptides. Methods Cell Biol 65:175–187
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Frydman J, Höhfeld J (1997) Chaperones get in touch: the hip-hop connection. Trends Biochem Sci 22:87–92
Fu D, Chen J, Zhang Y, Yu Z (2011) Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish Shellfish Immun 31:118–125
Geething MJ, Sambrook J (1992) Protein folding in the cell. Nature 355:33–45
Georgopoulos C, Welch WJ (1993) Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol 9:601–634
Gonzalez CRM, Bradley BP (1994) Are there salinity stress proteins? Mar Enivron Res 35:79–83
Guo JW, Xu ZW, Yu XJ, Qin SK, Ren PJ (2003) The tactics and reasons of the difficulty of larval abnormality. Sci Fish Cul 9:30–31 (in Chinese)
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98 NT. Nucl Acids Symp Ser 41:95–98
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–580
Hasday JD, Singh IS (2000) Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones 5:471–480
Henry RP, Wheatly MG (1992) Interaction of respiration, ion regulation, and acid–base balance in the everyday life of aquatic crustaceans. Am Zool 32:407–416
Hensold JO, Hunt CR, Calderwood SK, Housman DE, Kingston RE (1990) DNA binding of the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol Cell Biol 10:1600–1608
Huang WJ, Leu JH, Tsau MT, Chen JC, Chen LL (2011) Differential expression of LvHSP60 in shrimp in response to environmental stress. Fish Shellfish Immun 30:576–582
Ji DS (2005) Techniques of pond-farming of swimming crab, Portunus trituberculatus. Spe Econo Ani Plant 3:12–13 (in Chinese)
Jiang S, Xu Q (2011) The influence of salinity stress on the activity of gill Na+/K+-ATPase in swimming crab, Portunus trituberculatus. J Fishery Science 35:45–50 (in Chinese)
Kammenga JE, Arts MSJ, Oude-Breuil WJM (1998) HSP60 as a potential biomarker of toxic stress in the Nematode Plectus acuminatus. Arch Environ Contam Toxicol 34:253–258
Kozlova T, Perezgasga T, Reynaud E, Zurita M (1997) The Drosophila melanogaster homologue of the hsp60 gene is encoded by the essential locus 1 (1)10Ac and is differentially expressed during fly development. Dev Genes Evol 207:253–263
Krebs RA, Feder ME (1997) Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones 2:60–71
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, Gwadz M, Hao L, He S, Hurwitz DI, Jackson JD, Ke Z, Krylov D, Lanczycki CJ, Liebert CA, Liu C, Lu F, Lu S, Marchler GH, Mullokandov M, Song JS, Thanki N, Yamashita RA, Yin JJ, Zhang D, Bryant SH (2007) CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res 35:D237–D240
Meinhardt A, Wilhem B, Seitz J (1999) Expression of mitochondrial marker proteins during spermatogenesis. Human Reprod Update 5:108–119
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796
Niu C, Rummer J, Brauner C, Schulte P (2008) Heat shock protein (HSP 70) induced by mild heat shock inhibits sharp plasma osmolarity increases upon seawater transfer in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol 148 C:460–461
Nover L, Scharf KD (1997) Heat stress proteins and transcription factors. Cell Mol Life Sci 53:80–103
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Pearl LH, Prodromou C (2006) Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem 75:271–294
Pedersen SN, Lundebye AK (1996) Metallothionein and stress protein levels in shore crabs (Carcinus maenas) along a trace metal gradient in the Fal Estuary System (UK). Mar Environ Res 42:241–246
Péqueux A (1995) Osmotic regulation in crustaceans. J Crust Biol 15:1–60
Quintana FJ, Cohen IR (2005) Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol 175:2777–2782
Reddy PS, Thirulogachandar V, Vaishnavi CS, Aakrati A, Sopory SK, Reddy MK (2011) Molecular characterization and expression of a gene encoding cytosolic Hsp90 from Pennisetum glaucum and its role in abiotic stress adaptation. Gene 474:29–38
Rhee JS, Raisuddin S, Lee KW, Seo JS, Ki JS, Kim IC, Park HG, Lee JS (2009) Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp Biochem Physiol Part C 149:104–112
Sanchez GI, Carucci DJ, Sacci JJ, Resau JH, Rogers WO, Kumar N, Hoffman SL (1999) Plasmodium yoelli: cloning and characterization of the gene encoding for the mitochondrial heat shock protein 60. Exp Parasitol 93:181–190
Smurov A, Podlipaeva Y, Goodkov A (2007) Heat shock protein of the Hsp70 family in the euryhaline cilate Paramecium nephridiatum and its role in adaptation to salinity changes. Cell Tissue Biol 1:244–247
Spees JL, Chang SA, Snyder MJ, Chang ES (2002a) Thermal acclimation and stress in the American lobster, Homarus americanus: equivalent temperature shifts elicit unique gene expression patterns for molecular chaperones and polyunbiquitin. Cell Stress Chaperones 7:97–106
Spees JL, Chang SA, Snyder MJ, Chang ES (2002b) Osmotic induction of stress-responsive gene expression in the lobster Homarus americanus. Biol Bull 203:331–337
Sun YM (1984) Larval development of the swimming crab, Portunus trituberculatus. J Fish China 8:219–226 (in Chinese)
Taylor HH, Taylor EW (1992) Gills and lungs: the exchange of gases and ions. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates. Vol 10. Decapod Crustacea. Wiley, New York, pp 203–293
Terasawa K, Minami M, Minam Y (2005) Constantly updated knowledge of Hsp90. J Biochem 137:443–447
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Timakov B, Zhang P (2001) The hsp60B gene in Drosophila melanogaster is essential for the spermatid individualization process. Cell Stress Chaperones 6:71–77
Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Haucker H, Wagner H (2001) Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 276:31332–31339
Viant MR, Werner I, Rosenblum ES (2003) Correlation between heatshock protein induction and reducedmetabolic condition in juvenile steelhead trout (Oncorhynchus mykiss) chronically exposed to elevated temperature. Fish Physiol Biochem 29:159–171
Werner I, Nagel R (1997) Stress proteins HSP60 and HSP70 in three species of amphipods exposed to cadmium, diazinon, dieldrin, and fluoranthene. Environ Toxicol Chem 16:2393–2403
Xu Q, Liu Y (2011) Gene expression profiles of the swimming crab Portunus trituberculatus exposed to salinity stress. Mar Biol 158:2161–2172
Xu Q, Liu Y, Liu R (2010) Expressed sequence tags from cDNA library prepared from gills of the swimming crab, Portunus trituberculatus. J Exp Mar Biol Ecol 394:105–115
Xue J, Du N, Nai W (1997) The researches on the Portunus trituberculatus in China. Donghai Mar Sci 15:60–64 (in Chinese)
Zhou J, Wang W, He W, Zheng Y, Wang L, Xin Y, Liu Y, Wang A (2010) Expression of HSP60 and HSP70 in white shrimp, Litopenaeus vannamei in response to bacterial challenge. J Invertebr Pathol 103:170–178
Acknowledgments
This work is supported in part by grants from the National Natural Science Foundation of China (grant no. 30800840), and the Shanghai Young Rising Star of Science and Technology Program (grant no. 09QA1402600) to Qianghua Xu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Q., Qin, Y. Molecular cloning of heat shock protein 60 (PtHSP60) from Portunus trituberculatus and its expression response to salinity stress. Cell Stress and Chaperones 17, 589–601 (2012). https://doi.org/10.1007/s12192-012-0334-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-012-0334-6