Abstract
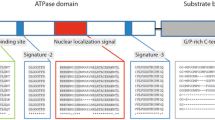
The techniques of homology cloning and anchored PCR were used to clone the Hsp90 gene from black tiger shrimp. The full length cDNA of black tiger shrimp Hsp90 (btsHsp90) contained a 5′ untranslated region (UTR) of 72 bp, an ORF (open reading frame) of 2160 bp encoding a polypeptide of 720 amino acids with an estimated molecular mass of 83-kDa and a 3′ UTR of 288 bp. The sequence of the coding region showed 90 and 84% homology with that of the Chiromantes haematocheir and Homo sapiens, respectively. Conserved signature sequences of Hsp90 gene family were found in the btsHsp90 deduced amino acid sequence. The temporal expressions of Hsp90 gene were constitutively in the black tiger shrimp tissues including liver, ovary, muscle, brain stomach, and heart, and their levels were markedly enhanced after 30-min heat treatment at 37°C. In ovarian maturation stages, the expression of btsHsp90 was strongest in the second stage, weaker in the fourth and first stage.





Similar content being viewed by others
References
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Liao F, Andalibi A, Qiao JH, Allayee H, Fogelman AM, Lusis AJ (1994) Genetic evidence for a common pathway mediating dative stress, inflammatory gene induction, and aortic fatty streak formation in mice. J Clin Invest 94:877–884
Benjamin IJ, Williams RS (1994) Expression and function of stress proteins in the ischemic heart. In: Morimoto RI, Tisserees A, Geogopoulos C (eds) The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 533–552
Mestril R, Dillmann WH (1995) Heat shock proteins and protection against myocardial ischemia. J Mol Cell Cardiol 27:45–52
Buchner J (1999) hsp90 and Co.—a holding for folding. Trends Biochem Sci 24:136–141
Craig EA, Weissman JS, Horwich AL (1994) Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell 78:365–372
Gress TM, Muller-Pillasch F, Weber C, Lerch MM, Freiss H, Buchler M, Beger HG, Adler G (1994) Balance of expression of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in chronic pancreatitis. Cancer Res 54:547–551
Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM (1994) Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA 91:8324–8328
Aligue R, Akhavan-Niak H, Russell P (1994) A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J 13:6099–6106
Srivastava PK, Udono H, Blachere NE, Li Z (1994) Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics 39:93–98
Jakob U, Buchner J (1994) Assisting spontaneity: the role of HSP90 and small HSPs as molecular chaperones. Trends Biochem Sci 19:205–211
Smith DF, Toft DO (1993) Steroid receptors and their associated proteins. Mol Endocrinol 7:4–11
Czar MJ, Welsh MJ, Pratt WB (1997) Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. J Biochem 36:7776–7785
Stancato LF, Chow YH, Hutchison KA, Perdew GH, Jove R, Pratt WB (1993) Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biochem 268:21711–21716
Sanchez FR, Toft DO, Schlesinger MJ, Pratt WB (1985) Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biochem 260:12398–13401
Czar MJ, Galigniana MD, Silverstein AM, Pratt WB (1996) Immunofluorescence localization of the 90-kDa heat-shock protein to cytoskeleton. Eur J Cell Biol 70:322–330
Minami Y, Kawasaki H, Miyata Y, Suzuki K, Yahara I (1991) Analysis of native forms and isoform compositions of the mouse 90-kDa heat shock protein, HSP90. J Biochem 266:10099–10103
Inanobe A, Takahashi K, Katada T (1994) Association of the beta gamma subunits of trimeric GTP-binding proteins with 90-kDa heat shock protein, hsp90. J Biochem 115:486–492
Csermely P, Schnaider T, Soti C, Proha´szka Z, Nardai G (1998) The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther 79:129–168
Voss AL, Thomas T, Gruss P (2000) Mice lacking hsp90_fail to develop a placental labyrinth. Development 127:1–11
Schlesinger MJ (1994) How the cell copes with stress and the function of heat shock proteins. Pediatr Res 36:1–6
Macario AJL (1995) Heat-shock proteins and molecular chaperones; implications for pathogenesis, diagnostics, and therapeutics. Int J Clin Lab Res 25:59–70
Georgopoulos C, Welch WJ (1993) Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol 9:601–634
Wang B, Li F-h, Dong B, Zhang X-j, Zhang C-s, Xiang J-h (2006) Discovery of the genes in response to white spot syndrome virus (WSSV) infection in Fenneropenaeus chinensis through cDNA microarray. Mar Biotechnol 8:491–500
Huang JH, Zhou FL, Ma ZM, Jiang SG (2005) Morphological and histological observation on ovary development of Penaeus monodon from northern South China Sea. J Trop Oceanogr 25(3):47–52
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTALX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Bevilacqua A, Ceriani MC, Capaccioli S, Nicolin A (2003) Posttranscriptional regulation of gene expression by degradation of messenger RNAs. J Cell Physiol 195:356–372
Zhang T, Kruys V, Huez G, Gueydan C (2002) AU-rich element mediated translational control: complexity and multiple activities of trans-activating factors. Biochem Soc Trans 30:952–958
Brunt SA, Silver JC (2004) Molecular cloning and characterization of two different cDNAs encoding the molecular chaperone Hsp90 in the Oomycete Achlya ambisexualis. Fungal Genet Biol 41:239–252
Rebbe NF, Ware J, Bertina RM, Modrich P, Stafford DW (1987) Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene 53:235–245
Hickey E, Brandon SE, Smale G, Lloyd D, Weber LA (1989) Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol Cell Biol 9:2615–2626
Anderson I, Brass A (1998) Searching DNA databases for similarities to DNA sequences: when is a match significant? Bioinformatics 14(4):349
Lees-Miller SP, Anderson CW (1989) The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biochem 264:17275–17280
Eggers DK, Welch WJ, Hansen WJ (1997) Complexes between nascent polypeptides and their molecular chaperones in the cytosol of mammalian cells. Mol Biol Cell 8:1559–1573
Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90:65–75
Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP (1997) Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89:239–250
Buchberger A, Bakau B (1997) Escherichia coli DnaK. In: Gething MJ (ed) Guidebook to molecular chaperones and protein-folding catalysts, vol. 147. Oxford University Press, Oxford
Ahn HJ, Kim S, Nam HW (2003) Molecular cloning of the 82-kDa heat shock protein (HSP90) of Toxoplasma gondii associated with the entry into and growth in host cells. Biochem Biophys Res Commun 311:654–659
Nathan DF, Vos MH, Lindquist S (1997) In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci USA 94:12949–12956
Quraishi H, Brown IR (1995) Expression of heat shock protein 90 (hsp90) in neural and nonneural tissues of the control and hyperthermic rabbit. Exp Cell Res 219:358–363
Huang HC, Yu JS, Tsay CC, Lin JH, Huang SY, Fang WT, Liu YC, Tzang BS, Lee WC (2002) Perification and characterization of porcine testis 90-kDa heat shock protein (Hsp90) as a substrate for various protein kinases. J Protein Chem 21:111–121
Huang H-c, Lee W-c, Lin J-h, Huang H-w, Jian S-c, Mao SJT, Yang P-c, Huang T-y, Liu Y-c (1999) Molecular cloning and characterization of porcine cDNA encoding a 90-kDa heat shock protein and its expression following hyperthermia. Gene 226:307–315
Acknowledgments
This study was supported by National nature foundation of China (No. 30571447), National “863” Project of China (No. 2003AA603120 ) and Agriculture Department Project of China(06-05-01B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, S., Qiu, L., Zhou, F. et al. Molecular cloning and expression analysis of a heat shock protein (Hsp90) gene from black tiger shrimp (Penaeus monodon). Mol Biol Rep 36, 127–134 (2009). https://doi.org/10.1007/s11033-007-9160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-007-9160-9