Abstract
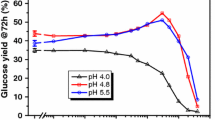
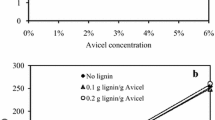
Enzymatic hydrolysis of lignocellulosic biomass is limited by rapid cellulase deactivation, consequently requiring large amounts of enzyme to maintain acceptable biomass conversion. In this study, a new approach to improve lignocellulose hydrolysis was investigated. Performing enzymatic hydrolysis of corn stover (CS) in the presence of polymeric–surfactant micelles (PMs) was demonstrated to improve hydrolysis yield to a greater extent than using only surfactant micelles. Application of 2 % (w/w) of polyethylene glycol (PEG 6000) with casein, Tween-20, and Triton X-100 at levels above the critical micelle concentrations increased the hydrolysis yield of CS containing high-bound lignin (extrusion-pretreated) by up to 87.8, 11.7, and 7.5 %, respectively. These PMs were not effective during enzymatic hydrolysis of biomass lacking lignin (Avicel) or alkali-pretreated CS (7.2 % lignin). The main reasons for the enhanced cellulase activity observed due to PEG-casein, PEG-Tween, and PEG-Triton were enhanced cellulase solubilization; reformation of α-helix substructure; and combination of induced cellulase solubilization, α-helix reformation, and chemical changes in the microstructure of biomass, respectively. Deformation of the cellulase substructure during hydrolysis of biomass and its subsequent reformation in the presence of surfactants were shown in this study for the first time. Chemical changes in the microstructure of biomass (e.g., lignin side changes, C–O bonds, and amorphous cellulose) were found to be another potential reason for the effectiveness of surfactants when they are incubated at above 6 g/L for 72 h with biomass.






Similar content being viewed by others
References
Kumar R, Wyman CE (2008) Effect of additives on the digestibility of corn stover solid following pretreatment by leading technologies. Biotechnol Bioeng 102:1544–1557
Taherzadeh MJ, Niklasson C, Lidén G (1999) Conversion of dilute-acid hydrolyzates of spruce and birch to ethanol by fed-batch fermentation. Bioresour Technol 69:59–66
Lee Y, Iyer P, Torget RW (1999) Dilute-acid hydrolysis of lignocellulosic biomass. Adv Biochem Eng/Biotechnol 65:93–115
Taherzadeh MJ, Eklund R, Gustafsson L, Niklasson C, Lidén G (1997) Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind Eng Chem Res 36:4659–4665
Wyman CE (1996) Handbook on bioethanol: production and utilization. Taylor & Francis, Washington, DC
Ferreira SMP, Duarte AP, Queiroz JA, Domingues FC (2009) Influence of buffer systems on Trichoderma reesei Rut C-30 morphology and cellulase production. Electron J Biotechnol 12:1–9
Ma AZ, Hu Q, Qu YB, Bai ZH, Liu WF, Zhuang GQ (2008) The enzymatic hydrolysis rate of cellulose decrease with irreversible adsorption of cellobiohydrolase I. Enzyme Microb Technol 42:543–547
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087. doi:10.1002/bit.24370
Kim S, Holtzapple MT (2006) Effect of structural features on enzyme digestibility of corn stover. Bioresour Technol 97:583–591
Zhuo YM, Kim TH, Lee YY, Chen RG, Elander RT (2006) Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. Appl Biochem Biotechnol 130:586–598
Eckard AD, Muthukumarappan K, Gibbons W (2011) Pretreatment of extruded corn stover with polyethylene glycol to enhance enzymatic hydrolysis: optimization, kinetics, and mechanism of action. BioEnerg Res 5:424–438
Zheng Y, Pan Z, Zhang R (2008) Non-ionic surfactants and noncatalytic protein treatment on enzymatic hydrolysis of pretreated creeping wild ryegrass. Appl Biochem Biotechnol 146:231–248
Stenberg K, Tengborg C, Galbe M, Zacchi G, Palmqvist E, HahnHagredal B (1998) Recycling of process stream in ethanol production from softwood based on enzymatic hydrolysis. Appl Biochem Biotechnol 70–72:697–708
Hoshino E, Tanaka A (2003) Enhancement of enzymatic catalysis of Bacillus amyloliquefaciens α-amylase by nonionic surfactant micelles. J Surfactant Deterg 6:299–303
Lindhoud S (2009) Polyelectrolyte complex micelles as wrapping for enzymes. Thesis, van Wageningen Universiteit
Maobing T, Zheng X, Paice M, McFarlane P, Saddler JN (2009) Effect of surfactants on separate hydrolysis fermentation and simultaneous saccharification and fermentation pretreated lodgepole pine. Biotechnol Prog 25:1122–1128
Helle SS, Duff SJB, Copper DG (1993) Effect of surfactants on cellulose hydrolysis. Biotechnol Bioeng 42:611–617
Castanon M, Wilke CR (1981) Effect of the surfactant Tween 80 on enzymatic hydrolysis of newspaper. Biotechnol Bioeng 23:1365–1372
Bin Y, Wyman CE (2006) BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrate. Wiley InterScience 94:611–617
Sangtian Y, An LI, Hao Z, Mingfang L, Xinhui X (2006) Effects of ionic surfactants on bacterial luciferase and α-amylase. Chin J Chem Eng 17:829–834
Szleifer I (1997) Polymers and proteins: interactions at interfaces. Curr Opin Solid State Mater Sci 2:337–344
Kim MH, Lee SB, Ryu DDY (1982) Surface deactivation of cellulose and its prevention. Enzyme Microb Technol 4:99–103
Kurakake M, Ooshima H, Kato J, Harano Y (1994) Pretreatment of bagasse by non-ionic surfactant for the enzymatic hydrolysis. Bioresour Technol 49:247–251
Qing Q, Yang B, Wyman CE (2010) Impact of surfactants on pretreatment of corn stover. Bioresour Technol 101:5941–5951
Seo DJ, Fujito H, Sakoda A (2011) Effects of a non-ionic surfactant, Tween 20, on adsorption/desorption of saccharification enzymes onto/from lignocelluloses and saccharification rate. Adsorption 17:813–822
Ahl PL et al (1997) Enhancement of the in vivo circulation lifetime of L-alpha-line liposomes: importance of liposomal aggregation versus complement opsonization. Biochim Biophys Acta 1329:370–382
Lu KW, Pérez-Gil J, Taeusch HW (2009) Kinematic viscosity of therapeutic pulmonary surfactants with added polymers. Biochim Biophys Acta 1788:632–637
Yamada Y, Kuboi R, Komasawa I (1993) Increased activity of Chromobacterium viscosum lipase in aerosol OT reverse micelles in the presence of nonionic surfactants. Biotechnol Prog 9:468–472
Hayashi Y, Talukder MMR, Wu J, Takeyama T, Kawanishi T, Shimizu NJ (2001) Increased activity of Chromobacterium viscosum lipase in AOT reverse micelles in the presence of short chain methoxypolyethylene glycol. J Chem Technol Biotechnol 76:844–850
Talunder MM, Takayama T, Hayashi Y, Wu JC, Kawanishi T, Shimizu N, Ogino C (2003) Improvement of enzyme activity and stability by adding of low molecular weight polyethylene glycol to sodium bis(2-ethyl-l-hexyl) sulfosuccinate/isooctane reverse micelles. Appl Biochem Biotechnol 110:101–111
Moghimi SM, Patel HM (1989) Serum opsonins and phagocytosis of saturated and unsaturated phospholipid liposomes. Biochim Biophys Acta 984:384–387
Sheth SR, Leckband D (1997) Measurements of attractive forces between proteins and end-grafted poly(ethylene glycol) chains. Proc Natl Acad Sci U S A 94:8399–8404
Vert M, Domurado D (2000) Poly(ethylene glycol): protein-repulsive or albumin-compatible. J Biomaterials Sci Polym Edn 11:1307–1317
Gupta R, Lee YY (2010) Pretreatment of corn stover and hybrid poplar by sodium hydroxide and hydrogen peroxide. Biotechnol Prog 26:1180–1186
Karunanithy C, Muthukumarappan K (2011) Optimization of corn stover and extruder parameters for enzymatic hydrolysis using response surface methodology. Biol Eng 3(2):73–95
Eckard AD, Muthukumarappan K, Gibbons W (2012) Modeling of pretreatment condition of extrusion pretreated prairie cordgrass and corn stover with polyoxyethylen (20) sorbitan monolaurate. Appl Biochem Biotechnol 167:377–393
Chundawat SP, Venkatesh B, Dale BE (2006) Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol Bioeng 96:219–231
Kumar R, Mago G, Balan V, Wyman CE (2009) Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour Technol 100:3948–3962
Dong A, Jones LS, Kewin BA, Krishnan S (2006) Secondary structure of proteins adsorbed onto aluminium hydroxide: infrared spectroscopic analysis of proteins from solution concentrations. Anal Biochem 351:282–289
Dong A, Huang P, Caughy WS (1994) Infrared methods for study of hemoglobin reactions and structures. Methods Enzymol 232:139–175
Dong A, Huang P, Caughy WS (1990) Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry 29:3303–3308
Kong J, YU S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39:549–559
Biasutti MA, Abuin EB, Silber JJ, Correa NM, Lissi EA (2008) Kinetics of reactions catalyzed by enzymes in solutions of surfactants. Adv Colloid Interface Sci 136:1–24
Chen N, Fan JB, Xiang J, Chen J, Liang Y (2006) Enzymatic hydrolysis of microcrystalline cellulose in reverse micelles. Biochim Biophys Acta 1764:1029–1035
Rahman RNZA, Geok LP, Basri M, Salleh AB (2006) An organic solvent-stable alkaline protease from Pseudomonas aeruginosa strain K: enzyme purification and characterization. Enzym Microb Technol 39:1484–1491
Geok LP, Nyonya C, Abdul Razak CN, Abd Rahman RNZ, Basri M, Salleh AB (2003) Isolation and screening of an extracellular organic solvent-tolerant protease producer. Biochem Eng J 13:73–77
Liu H, Zhu JY (2010) Eliminating inhibition of enzymatic hydrolysis by lignosulfonate in unwashed sulfite-pretreated aspen using metal salts. Bioresour Technol 101:9120–9127
Li J, Li S, Fan C, Yan Z (2011) The mechanism of poly(ethylene glycol) 4000 effect on enzymatic hydrolysis of lignocellulose. Colloid Surf B: Biointerfaces 89:203–120
Ericksson T, Börjesson J, Tjerneld F (2002) Mechanism of surfactant effect on enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 31:353–364
Abuin EB, Scaiano JC (1984) Exploratory study of the effect of polyelectrolyte surfactant aggregates on photochemical behavior. J Am Chem Soc 106:6274
Woodle MC, Lasic DD (1992) Sterically stabilized liposomes. Biochim Biophys Acta 111(3):171–199
Lee JH, Lee HB, Andrade JD (1995) Blood compatibility of polyethylene oxide surfaces. Prog Polym Sci 20:1043–1079
Molineux G (2002) Pegylation: engineering improved pharmaceuticals for enhanced therapy. Cancer Treat Rev 28:13–16
Tirosh O, Barenholz Y, Katzhender J, Priev A (1998) Hydration of polyethylene glycol-grafted liposomes. Biophys J 74:1371–1379
Leiwa OW, Quina FH (1994) Photophysical probe studies of polymer–detergent interactions. J Braz Chem 6:173–178
Guo W, Sun YW, Luo GS, Wang YJ (2005) Interaction of PEG with ionic surfactant SDS to form template for mesoporous material. Colloids Surf A: Physicochem Eng Asp 252:7–77
Zhang Y, Zhang Y, Tang L (2011) Effect of PEG 4000 on cellulose catalysis in the lignocellulose saccharification process. J Chem Technol Biotechnol 86:115–120
Sun XF, Xu F, Sun RC, Fowler P, Baird MS (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr Res 340:97–106
Faix O, Beinhoff O (1988) FTIR spectra of milled wood lignins and lignin polymer models (DHP's) with enhanced resolution obtained by deconvolution. J Wood Chem Technol 8:505–522
Kristensen JB, Thygesen LG, Felby C, Jorgensen H, Elder T (2008) Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol Biofuels 1:5–13
Stewart D, Yahiaoui N, McDougall GJ, Myton K, Marque C, Boudet AM, Haigh J (1997) Fourier transform infrared and Raman spectroscopic evidence for the incorporation of cinnamaldehydes into the lignin of transgenic tobacco (Nicotiana tabacum L.) plants with reduced expression of cinnamyl alcohol dehydrogenase. Planta 201:311–318
Kaar WK, Holtzapple MT (1998) Benefits from Tween during enzymatic hydrolysis of corn stover. Biotechnol Bioeng 59:419–427
Börjesson J, Engqvist M, Slipos B, Tjerneld F (2007) Effect of polyethylene glycol on enzymatic hydrolysis and adsorption of cellulose enzymes to pretreated lignocellulose. Enzyme Microb Technol 41:186–195
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv Colloid Interface Sci 110:75–95
Viparelli P, Alfani F, Cantarella M (1999) Models for enzyme superactivity in aqueous solutions of surfactants. Biochem J 344:765–773
Mizutani C, Sethumadhavan K, Howley P (2002) Effect of a nonionic surfactant on Trichoderma cellulase treatments of regenerated cellulose and cotton yarns. Cellulose 9:83–89
Roach LA (2009) The casein micelle as an encapsulation system for triclosan: methods of micelle dissociation, encapsulation, release, and in vitro delivery. University of Tennessee–Knoxville
Sonati S, Appu Rao AG (1993) Kinetic and structural studies on the interaction of surfactants with lipoxygenase L1 from soybeans (Glycine max). J Agric Food Chem 41:366–371
Xiang J, Fan JB, Chen N, Liang CY (2006) Interaction of cellulase with sodium dodecyl sulfate at critical micelle concentration level. Colloids Surf B Biointerfaces 49:175–180
Acknowledgments
The authors thank the Agricultural Experiment Station, South Dakota State University, and the North Central Sun Grant Center, Brookings, South Dakota, and US Department of Energy, Golden, CO (grant no. DE-FG36-08GO88073) for funding, facilities, equipment and supplies.
Conflict of interest
The authors of this work have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eckard, A.D., Muthukumarappan, K. & Gibbons, W. The Role of Polymeric Micelles on Chemical Changes of Pretreated Corn Stover, Cellulase Structure, and Adsorption. Bioenerg. Res. 7, 389–407 (2014). https://doi.org/10.1007/s12155-013-9379-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9379-3