Abstract
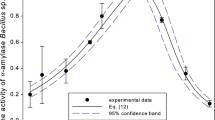
Some enzymes are considerably more stable when formulated with nonionic surfactants than when formulated with anionic surfactants. The effect of a nonionic surfatant, polyoxyethylene mono-N-dodecyl ether (Brij 35; number of units of ethylene oxide moieties, 23), on the kinetic behavior of hydrolysis of amylopectin with Bacillus amyloliquefaciens α-amylase (BAA) was studied at a temperature of 25°C and a pH of 7.0. The hydrolytic rate was accelerated by the addition of the nonionic surfactant above its critical micelle concentration. Lineweaver-Burk plots for the enzymatic hydrolysis in the absence and presence of the nonionic surfactant at 0.5 to 2.5% (wt/vol) had linear relationships, and the kinetic parameters, K m and k cat were obtained. The value of k cat was increased with an increased concentration of Brij 35, whereas the K m value was approximately constant. Therefore, the increase in k cat contributed to the acceleration of the apparent hydrolytic rate. The interaction of amylopectin with the surfactant was examined by a surface tension measurement, and the result confirmed the corresponding binding between the substrate and the surfactant. A fluorescence analysis due to tryptophan in BAA suggested that BAA bound to the nonionic micelles. The increase in k cat suggested that hydrolytic catalysis at the micellar pseudophase was more efficient than that at the aqueous pseudophase. The enhancement of the catalytic rate contributed to the effective removal of food stains containing starch when BAA was added with Brij 35 in a laundry detergent washing test.
Similar content being viewed by others
Abbreviations
- BAA:
-
α-amylase from Bacillus amyloliquefaciens
- Brij 35:
-
polyoxyethylene mono-N-dodecyl ether
- CMC:
-
critical micelle concentration
- ES complex:
-
enzyme-substrate complex
References
Kravetz, L., and K.F. Guin, Effect of Surfactant Structure on Stability of Enzymes Formulated into Laundry Liquids, J. Am. Oil Chem. Soc. 62:943 (1985).
Schomaecker, R., B.H. Robinson, and P.D.I. Fletcher, Interaction of Enzymes with Surfactants in Aqueous Solution and in Water-in-Oil Microemulsions, J. Chem. Soc. Faraday Trans. 1 84:4203 (1988).
Nakahara, H., S. Okada, K. Mochida, H. Ohmori, and M. Masui, Kinetic Studies on Pancreatic Lipase Activity in Micellar Systems, Chem. Pharm. Bull. 31:4213 (1983).
Tanaka, A., and E. Hoshino, Thermodynamic and Activation Parameters for the Hydrolysis of Amylose with Bacillus α-Amylases in a Diluted Anionic Surfactant Solution, J. Biosci. Bioeng. 93:485 (2002).
Montserret, R., M.J. McLeish, A. Böckmann, C. Geourjon, and F. Penin, Involvement of Electrostatic Interactions in the Mechanism of Peptide Folding Induced by Sodium Dodecyl Sulfate Binding, Biochemistry 39:8362 (2000).
Hagihara, Y., D.-P. Hong, M. Hoshino, K. Enjyoji, H. Kato, and Y. Goto, Aggregation of β2-Glycoprotein I Induced by Sodium Lauryl Sulfate and Lysophospholopids, Biochemistry 41:1020 (2002).
Russell, G.L., and L.N. Britton, Use of Certain Alcohol Ethoxylates to Maintain Protease Stability in the Presence of Anionic Surfactants, J. Surfact. Deterg. 5:5 (2002).
Martinek, K., A.V. Levashov, N. Klyachko, Y.L. Khmelnitski, and I.V. Berezin, Micellar Enzymology, Eur. J. Biochem. 155:453 (1986).
Robyt, J.F., and W.J. Whelan, The α-Amylases, in Starch and Its Derivatives, edited by J.A. Radley, 4th edn., Chapman & Hall, London, 1968, p. 430.
Takagi, T., H. Toda, and T. Isemura, Bacterial and Mold Amylases, in The Enzymes, edited by P.D. Boyer, Academic Press, New York, 1971, p. 235.
Fogarty, W.M., and C.T. Kelly, Amylases, Amyloglucosidases and Related Glucanases, in Microbial Enzymes and Bioconversions, edited by A.H. Rose, Academic Press, London, 1980, p. 115.
Kindle, K.L., Characteristics and Production of Thermostable α-Amylase, Appl. Biochem. Biotechnol. 8:153 (1983).
Kulp, K., Carbohydrases, in Enzymes in Food Processing, edited by G. Reed, 2nd edn., Academic Press, New York, 1975, p. 53.
Reichelt, J.R. Industrial Applications, in Industrial Enzymology, edited by T. Godfrey, and J.R. Reichelt, Nature Press, New York, 1983, p. 375.
Takkinen, K., R.F. Pettersson, N. Kalkkinen, I. Palva, H. Soderlund, and L. Kaariainen, Amino Acid Sequence of α-Amylase from Bacillus amyloliquefaciens Deduced from the Nucleotide Sequence of the Cloned Gene, J. Biol. Chem. 258:1007 (1983).
Hiromi, K., Interpretation of Dependency of Rate Parameters on the Degree of Polymerization of Substrate in Enzyme-Catalyzed Reactions. Evaluation of Subsite Affinities of Exoenzyme, Biochem. Biophys. Res. Commun. 40:1 (1970).
Thoma, J.A., C. Brothers, and J. Spradlin, Subsite Mapping of Enzymes. Studies on Bacillus subtilis Amylase, Biochemistry 9:1768 (1970).
Fitter, J., R. Herrmann, N.A. Dencher, A. Blume, and T. Hauss, Activity and Stability of a Thermostable α-Amylase Compared to Its Mesophilic Homologue: Mechanisms of Thermal Adaptation, Biochemistry 40:10723 (2001).
MacGregor, E.A., α-Amylase Structure and Activity, J. Protein Chem. 7:399 (1988).
Tanaka, A., and E. Hoshino, Study on the Substrate Specificity of α-Amylases That Contribute to Soil Removal in Detergent, J. Surfact. Deterg. 2:193 (1999).
Viparelli, P., F. Alfani, and M. Cantarella, Models for Enzyme Superactivity in Aqueous Solutions of Surfactants, Biochem. J. 344:765 (1999).
Svensson, E., M. Gudmundsson, and A.-C. Eliasson, Binding of Sodium Dodecylsulphate to Starch Polysaccharides Quantified by Surface Tension Measurements, Colloids Surf. B 6:227 (1996).
wulff, G., and S. Kubik, Circular Dichroism and Ultraviolet Spectroscopy of Complexes of Amylose, Carbohydr. Res. 237:1 (1992).
Hui, Y., J.C. Russell, and D.G. Whitten, Host-Guest Interactions in Amylose Inclusion Complexes, J. Am. Chem. Soc. 105: 1374 (1983).
Yamamoto, M., S. Harada, T. Nakatsuka, and T. Sano, Hydrodynamic Characterization of Amylose-SDS Complex by Viscosity Measurement, Bull. Chem. Soc. Jpn. 61:1471 (1988).
Burstein, E.A., N.S. Vedenkina, and M.N. Ivkova, Fluorescence and the Location of Tryptophan Residues in Protein Molecules, Photochem. Photobiol. 18:263 (1973).
Suzuki, A., T. Yamane, Y. Ito, T. Nishio, H. Fujiwara, and T. Ashida, Crystallization and Preliminary Crystallographic Study of Bacterial α-Amylases, J. Biochem. 108:379 (1990).
Bernfeld, P., Amylases, α and β, Methods Enzymol 1:149 (1955).
Padday, J.F., The Measurement of Surface Tension, in Surface and Colloid Science Vol. 1, edited by E. Matijevic, Wiley-Interscience, New York, 1969, p. 101.
Fendler, J.H., and E.J. Fendler, Principles of Micellar Catalysis in Aqueous Solutions, in Catalysis in Micellar and Macromolecular Systems, Academic Press, London, 1975, p. 86.
Torgerson, E.M., L.C. Brewer, and J.A. Thoma, Subsite Mapping of Enzymes. Use of Subsite Map to Simulate Complete Time Course of Hydrolysis of a Polymeric Substrate, Arch. Biochem. Biophys. 196:13 (1979).
Dua, R.D., and S. Kochhar, Active Site Studies on Bacillus amyloliquefaciens α-Amylase, Mol. Cell. Biochem. 66:13 (1985)
Ooshima, H., M. Sakata, and Y. Harano, Enhancement of Enzymatic Hydrolysis of Cellulose by Surfactant, Biotech. Bioeng. 28:1727 (1986).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hoshino, E., Tanaka, A. Enhancement of enzymatic catalysis of Bacillus amyloliquefaciens α-amylase by nonionic surfactant micelles. J Surfact Deterg 6, 299–303 (2003). https://doi.org/10.1007/s11743-003-0273-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11743-003-0273-2