Abstract
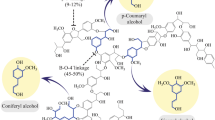
Independent down-regulation of genes encoding p-coumarate 3-hydroxylase (C3H) and hydroxycinnamoyl CoA:shikimate/quinate hydroxycinnamoyl transferase (HCT) has been previously shown to reduce the recalcitrance of alfalfa and thereby improve the release of fermentable sugars during enzymatic hydrolysis. In this study, ball-milled lignins were isolated from wild-type control, C3H, and HCT gene down-regulated alfalfa plants. One- and two-dimensional nuclear magnetic resonance (NMR) techniques were utilized to determine structural changes in the ball-milled alfalfa lignins resulting from this genetic engineering. After C3H and HCT gene down-regulation, significant structural changes had occurred to the alfalfa ball-milled lignins compared to the wild-type control. A substantial increase in p-hydroxyphenyl units was observed in the transgenic alfalfa ball-milled lignins as well as a concomitant decrease in guaiacyl and syringyl units. Two-dimensional 13C–1H heteronuclear single quantum coherence correlation NMR, one-dimensional distortionless enhancement by polarization transfer-135, and 13C NMR measurement showed a noteworthy decrease in methoxyl group and β-O-4 linkage contents in these transgenic alfalfa lignins. 13C NMR analysis estimated that C3H gene down-regulation reduced the methoxyl content by ~55–58% in the ball-milled lignin, while HCT down-regulation decreased methoxyl content by ~73%. The gene down-regulated C3H and HCT transgenic alfalfa lignin was largely a p-hydroxyphenyl (H) rich type lignin. Compared to the wild-type plant, the C3H and HCT transgenic lines had an increase in relative abundance of phenylcoumaran and resinol in the ball-milled lignins.








Similar content being viewed by others
Abbreviations
- C3H:
-
p-Coumarate 3-hydroxylase
- HCT:
-
Hydroxycinnamoyl CoA:shikimate/quinate hydroxycinnamoyl transferase
- NMR:
-
Nuclear magnetic resonance
- HSQC:
-
Heteronuclear single quantum coherence
- DEPT:
-
Distortionless enhancement by polarization transfer
- WT:
-
Wild-type
- /Ar:
-
Per aromatic ring
- H:
-
p-Hydroxyphenyl
- G:
-
Guaiacyl
- S:
-
Syringyl
References
Ragauskas AJ, Williams CK, Davison BH et al (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Horvath IT, Anastas PT (2007) Innovations and green chemistry. Chem Rev 107:2169–2173
Holdren JP (2007) Energy and sustainability. Science 315:737
Hoffert MI, Caldeira K, Benford G et al (2002) Advanced technology paths to global climate stability: energy for a greenhouse planet. Science 298:981–987
Chow J, Kopp RJ, Portney PR (2003) Energy resources and global development. Science 302:1528–1531
Clark JH, Budarin V, Deswarte FEI et al (2006) Green chemistry and the biorefinery: a partnership for a sustainable future. Green Chem 8:853–860
Pu Y, Zhang D, Singh PM, Ragauskas AJ (2008) The new forestry biofuels sector. Biofuels Bioproducts Biorefining 2:58–73
Ragauskas AJ, Nagy M, Kim DH et al (2006) From wood to fuels integrating biofuels and pulp production. Ind Biotechnol 2:55–65
Schell DJ, Riley CJ, Dowe N et al (2004) A bioethanol process development unit: initial operating experiences and results with a corn fiber feedstock. Bioresour Technol 91:179–188
Eggeman T, Elander RT (2005) Process and economic analysis of pretreatment technologies. Bioresour Technol 96:2019–2025
Lynd LR, Wyman CE, Gerngross TU (1995) Biocommodity engineering. Biotechnol Prog 15:777–793
Himmel ME, Ding SY, Johnson DK et al (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–807
Lynd LR, Laser MS, Bransby D et al (2008) How biotech can transform biofuels. Nat Biotechnol 26:169–172
Yang B, Gray MC, Liu C et al (2004) Unconventional relationships for hemicellulose hydrolysis and subsequent cellulose digestion. In: Saha BS, Hayashi K (eds) Lignocellulose biodegradation. ACS symposium series 889. American Chemical Society, Washington, pp 100–125
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioproducts Biorefining 2:26–40
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M et al (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Glasser WG, Wright RS (1998) Steam-assisted biomass fractionation II fractionation behavior of various biomass resources. Biomass Bioenergy 14:219–235
Ballesteros I, Negro MJ, Oliva JM, Cabanas A, Manzanares P, Ballesteros M (2006) Ethanol production from steam-explosion pretreated wheat straw. Appl Biochem Biotechnol 129–132:496–508
Liu C, Wyman CE (2004) Impact of fluid velocity on hot water only pretreatment of corn stover in a flowthrough reactor. Appl Biochem Biotechnol 113–116:977–987
Allen SG, Spencer MJ, Antal MJ Jr, Laser MS, Lynd LR (1997) Hot liquid water pretreatment of lignocellulosics at high solids concentrations. In: Bridgwater AV, Boocock DGB (eds) Developments in thermochemical biomass conversion, vol 1. Blackie, London, pp 765–772
van Walsum GP, Allen SG, Spencer MJ, Laser MS, Antal MJ, Lynd LR (1996) Conversion of lignocellulosics pretreated with liquid hot water to ethanol. Appl Biochem Biotechnol 57(58):157–170
Lloyd TA, Wyman CE (2005) Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol 96:1967–1977
Yang B, Wyman CE (2004) Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol Bioeng 86(1):88–95
Kim SB, Lee YY (2002) Diffusion of sulfuric acid within lignocellulosic biomass particles and its impact on dilute-acid pretreatment. Bioresour Technol 83:165–171
Chang VS, Nagwani M, Holtzapple MT (1998) Lime pretreatment of crop residues bagasse and wheat straw. Appl Biochem Biotechnol 74:135–159
Chang VS, Nagwani M, Kim CH, Holtzapple MT (2001) Oxidative lime pretreatment of high-lignin biomass: poplar wood and newspaper. Appl Biochem Biotechnol 94:1–28
Kim TH, Lee YY (2005) Pretreatment and fractionation of corn stover by ammonia recycle percolation process. Bioresour Technol 96:2007–2013
Teymouri F, Laureano-Perez L, Alizadeh H, Dale BE (2005) Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour Technol 96:2014–2018
Pan X, Kadla JF, Ehara K, Gilkes N, Saddler JN (2006) Organosolv ethanol lignin from hybrid poplar as a radical scavenger: relationship between lignin structure extraction conditions and antioxidant activity. J Agric Food Chem 54:5806–5813
Hasegawa I, Tabata K, Okuma O, Mae K (2004) New pretreatment methods combining a hot water treatment and water/acetone extraction for thermo-chemical conversion of biomass. Energy Fuels 18:755–760
Dale BE (2008) Biofuels: thinking clearly about the issues. J Agric Food Chem 56:3885–3891
Wyman CE (2008) Cellulosic ethanol: a unique sustainable liquid transportation fuel. MRS Bull 33:381–383
Huntley SK, Ellis D, Gilbert M, Chapple C, Mansfield SD (2003) Significant increases in pulping efficiency in C4H–F5H-transformed poplars: Improved chemical savings and reduced environmental toxins. J Agric Food Chem 51:6178–6183
Pilate G, Guiney E, Holt K, Petit-Conil M, Lapierre C, Leple JC et al (2002) Field and pulping performances of transgenic trees with altered lignification. Nat Biotechnol 20:607–612
Lapierre C, Pollet B, Petit-Conil M et al (1999) Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol 119:153–163
Davison BH, Drescher SR, Tuskan GA, Davis MF, Nghiem NP (2006) Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl Biochem Biotechnol 129–132:427–435
Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25:759–761
Jackson LA, Shadle GL, Zhou R et al (2008) Improving saccharification efficency of alfalfa stems through modification of the terminal stages of monolignol biosynthesis. Bioenerg Res 1:180–192
Reddy MSS, Chen F, Shadle G, Jackson L, Aljoe H, Dixon RA (2005) Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proc Natl Acad Sci USA 102:16573–16578
Chen F, Reddy MSS, Temple S, Jackson L, Shadle G, Dixon RA (2006) Multi-site genetic modulation of monolignol biosynthesis suggests new routes for formation of syringyl lignin and wall-bound ferulic acid in alfalfa (Medicago sativa L.). Plant J 48(1):113–124
Holtman KM, Chang H, Jameel H, Kadla J (2006) Quantitative 13C NMR characterization of milled wood lignins isolated by different milling techniques. J Wood Chem Technol 26:21–34
Ikeda T, Holtman K, Kadla JF, Chang H, Jameel H (2002) Studies on the effect of ball milling on lignin structure using a modified DFRC method. J Agric Food Chem 50:129–135
Bjorkman A (1956) Finely divided wood. I. Extraction of lignin with neutral solvents. Svensk Papperstidn 59:477–485
Hallac B, Sannigrahi P, Pu Y, Ray M, Murphy RJ, Ragauskas AJ (2009) Biomass characterization of Buddleja davidii: a potential feedstock for biofuel production. J Agric Food Chem 57:1275–1281
Ralph J, Akiyama T, Kim H et al (2006) Effects of coumarate 3-hydroxylase down-regulation on lignin structure. J Biol Chem 281:8843–8853
Rencoret J, Marques G, Gutierrez A, Ibarra D, Li J, Gellerstedt G et al (2008) Structural characterization of milled wood lignins from different eucalypt species. Holzforschung 62:514–526
del Rio JC, Rencoret J, Marques G, Gutierrez A, Ibarra D, Santos JI et al (2008) Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J Agric Food Chem 56:9525–9534
Stewart JJ, Akiyama T, Chapple C, Ralph J, Mansfield SD (2009) The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol 150:621–635
Pu Y, Ragauskas AJ (2005) Structural analysis of acetylated hardwood lignins and their photoyellowing properties. Can J Chem 83:2132–2139
Robert D (1992) Carbon-13 nuclear magnetic resonance spectrometry. In: Lin SY, Dence CW (eds) Methods in lignin chemistry. Springer, New York, pp 250–273
Ralph SA, Ralph J, Landucci LL (2004) NMR database of lignin and cell wall model compounds. http://ars.usda.gov/Services/docs.htm?docid=10491. Accessed 15 March 2009
Capanema EA, Balakshin MY, Kadla JF (2005) Quantitative characterization of a hardwood milled wood lignin by nuclear magnetic resonance spectroscopy. J Agric Food Chem 53:9639–9649
Zhang L, Gellerstedt G (2000) Achieving quantitative assignment of lignin structure by combining 13C and HSQC NMR technologies. In: Proceedings of sixth European workshop on lignocellulosics and pulp, Bordeaux, France, 3–6 September 2000
Zhang L, Gellerstedt G (2008) 2D heteronuclear (1H–13C) single quantum correlation (HSQC) NMR analysis of Norway spruce bark components. In: Hu TQ (ed) Characterization of lignocellulosic materials. Blackwell, Oxford, pp 3–6
Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C et al (2004) Silencing of hydroxycinnamoyl-coenzyme a shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell 16(6):1446–1465
Wagner A, Ralph J, Akiyama T, Flint H, Phillips L, Torr K et al (2007) Exploring lignification in conifers by silencing hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyltransferase in Pinus radiata. Proc Natl Acad Sci USA 104(28):11856–11861
Acknowledgment
We thank Drs. Richard A. Dixon and Mark Davis for their suggestion of the manuscript. The BioEnergy Science Center (BESC) is a US Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science. The authors would like to gratefully acknowledge the financial support from DOE Office of Biological and Environmental Research through the BioEnergy Science Center (DE-AC05-00OR22725).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Wild type and transgenic alfalfa plants (PDF 1221 kb).
ESM Fig. 1
Sugar release performance for alfalfa biomass subject to enzymatic hydrolysis (total sugar released as a percentage of total sugar in the cell wall residue) [37]. WT wild type control, filled biomass without acid pretreatment, blank biomass that was first acid pretreated (PDF 1221 kb).
Rights and permissions
About this article
Cite this article
Pu, Y., Chen, F., Ziebell, A. et al. NMR Characterization of C3H and HCT Down-Regulated Alfalfa Lignin. Bioenerg. Res. 2, 198–208 (2009). https://doi.org/10.1007/s12155-009-9056-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-009-9056-8