Abstract
Levels of amyloid-beta monomer and deposited amyloid-beta in the Alzheimer’s disease brain are orders of magnitude greater than soluble amyloid-beta oligomer levels. Monomeric amyloid-beta has no known direct toxicity. Insoluble fibrillar amyloid-beta has been proposed to be an in vivo mechanism for removal of soluble amyloid-beta and exhibits relatively low toxicity. In contrast, soluble amyloid-beta oligomers are widely reported to be the most toxic amyloid-beta form, both causing acute synaptotoxicity and inducing neurodegenerative processes. None of the amyloid-beta immunotherapies currently in clinical development selectively target soluble amyloid-beta oligomers, and their lack of efficacy is not unexpected considering their selectivity for monomeric or fibrillar amyloid-beta (or both) rather than soluble amyloid-beta oligomers. Because they exhibit acute, memory-compromising synaptic toxicity and induce chronic neurodegenerative toxicity and because they exist at very low in vivo levels in the Alzheimer’s disease brain, soluble amyloid-beta oligomers constitute an optimal immunotherapeutic target that should be pursued more aggressively.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, accounting for 60% to 80% of all dementias [1, 2]. Worldwide, the prevalence of dementia was more than 35 million in 2010, and projections exceed 65 million by 2030 and 115 million by 2050 [1]. Significantly, no drugs that prevent or slow the progression of AD are currently approved. The development of effective AD therapeutics is clearly a tremendous medical challenge and should be one of society’s top medical priorities.
Despite the great need and significant societal and financial incentives, many pharmaceutical companies and investors have reduced investments in the search for new AD drugs, citing recent clinical failures of several high-profile experimental AD therapeutics and the high risks and costs of such development endeavors. The recent clinical failures also have intensified scrutiny of the ‘amyloid cascade hypothesis’, which spawned many of the recent experimental AD drugs targeting the amyloid-beta (Aβ) peptide. Nevertheless, the causal linkage between Aβ and AD remains strong and is supported by hundreds of studies over the past two decades [3–10]. (This is a representative sample of published reviews, and apologies are given to the authors of many excellent reviews that are not cited.)
Essentially all Aβ therapeutic approaches so far have targeted reducing the levels of Aβ monomer or Aβ deposits (or both) in the brain. However, today, the causal role of Aβ in AD is widely considered to involve soluble Aβ oligomers, and therapeutic strategies that selectively target soluble Aβ oligomers offer the potential to deliver rapid symptomatic benefit and long-term disease modification. This review describes the role of soluble Aβ oligomers within the amyloid hypothesis and discusses implications for current Aβ immunotherapies and new immunotherapies directed selectively toward soluble Aβ oligomers.
The amyloid cascade hypothesis
The first suggestion of an ‘amyloid hypothesis’ to explain the pathology of AD was that of Wong and colleagues [11], who postulated that Aβ-derived cerebrovascular amyloid caused seepage of Aβ and other substances from plasma into the brain, leading to the formation of Aβ plaques and possibly neurodegeneration. This was revised into the more well-known amyloid cascade hypothesis that proposed that deposition of Aβ as neuritic plaques caused AD and led to neurofibrillary tangles, cell loss, vascular damage, and dementia [12]. The amyloid hypothesis linking Aβ to AD catalyzed much of AD and Aβ research over the past two decades, and key studies during that period led to important revisions of the hypothesis that highlighted the central role of soluble Aβ oligomers in synaptic dysfunction and loss [4, 13–19].
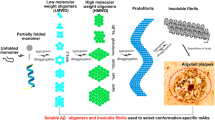
The current understanding of the Aβ cascade is derived primarily from in vitro studies, the vast majority of which were conducted by using Aβ concentrations orders of magnitude greater than those found in vivo. The assembly of Aβ peptides to form soluble oligomers, protofibrils, and fibrils is well documented to be affected by the isoform of the starting peptide, how the peptide is treated prior to assembly, the nature of the buffer, pH, peptide concentration, assembly temperature and time, agitation, and presence of other peptides or biological materials [20–24]. Moreover, preparations of soluble Aβ species have been shown to change with time or upon dilution in different buffers, particularly in cell culture media [19]. Thus, caution must be taken in extrapolating the results and conclusions of in vitro studies to in vivo reality. Although precise mechanistic details remain to be elucidated, a multitude of studies by numerous researchers support the conclusion that monomeric Aβ peptides assemble to form soluble Aβ oligomers, which further aggregate to form fibrillar Aβ [17, 25].
Three distinct pools of Aβ species exist: Aβ monomers, soluble Aβ oligomers, and insoluble fibrillar Aβ. Each of these pools encompasses an array of individual species. Thus, monomeric Aβ peptides encompass various isoforms, including Aβ(1-40), Aβ(1-42), and Aβ(1-43), as well as numerous N-terminal truncated isoforms. (For example, see the introductory paragraphs of Tekirian and colleagues [26].) Insoluble fibrillar Aβ aggregates are also known to be heterogeneous in structure and composed of various Aβ isoforms, both full-length as well as N-terminal and C-terminal truncated isoforms. (For example, see the introductory paragraphs of Roher and colleagues [27] and Thal and colleagues [28].)
Soluble Aβ oligomers are also heterogeneous and perhaps more ambiguous because of the different terminologies used by different researchers to describe them. (For an excellent review of soluble Aβ oligomers, see Benilova and colleagues [9].) Thus, soluble Aβ oligomer species reported by various researchers have been termed sodium dodecyl sulfate (SDS)-stable Aβ oligomers [29, 30], low-n-oligomers [31–33], dimers [33–35], trimers [33, 36–38], tetramers [37], paranuclei [38, 39], dodecamers and Aβ*56 [37, 40, 41], amyloid-derived diffusible ligands (ADDLs) [42–44], Aβ oligomers [45], prefibrillar oligomers [46], Aβ globulomers [40, 47–49], spherical oligomers [50], amylospheroids [51, 52], protofibrils [20, 53, 54], and annular protofibrils [55, 56]. Most of these terminologies refer to a mixture of metastable, soluble Aβ oligomer species in equilibrium rather than a discrete, stable species. In many cases, there is similarity in the species comprising the different preparations. In this review, we will use the terminology soluble Aβ oligomers to describe Aβ species composed of more than one Aβ peptide that remain in solution following centrifugation. Aβ fibrils or fibrillar Aβ will be used as a general description of insoluble Aβ plaques and vascular amyloid. (The term Aβ plaques rather than amyloid plaques will be used to more precisely describe amyloid plaques comprising primarily Aβ peptides versus those comprising primarily non-Aβ peptides.)
It has been established that the levels of Aβ monomer and deposited fibrillar Aβ in the AD brain are orders of magnitude greater than soluble Aβ oligomer levels [57–65]. However, more than three decades of intense investigation has not provided a precise understanding of the extent of interconversion among the various Aβ species.
Aβ dimers have been demonstrated to form at physiologically relevant concentrations of Aβ monomer in vivo[66, 67]. Numerous in vitro studies have shown that soluble Aβ oligomers and protofibrils form under similar conditions and that both species can proceed to form larger fibrillar species [20, 43, 53, 68–74]. More recent studies provide further support for the addition of soluble Aβ oligomers to protofibrillar and fibrillar assemblies and also provide data indicating that oligomerization can occur via a secondary nucleation mechanism caused by fibrillar Aβ [73, 74], possibly suggesting a mechanistic linkage between fibrillar Aβ and soluble Aβ oligomers. However, the precise mechanism of in vivo fibril formation has not been fully established.
Aβ plaques are now generally considered to be a relatively benign species [19, 72, 75]; however, whether Aβ plaques are a sink or a source for toxic soluble Aβ oligomers is a subject of debate. Several studies with γ-secretase inhibitors [76–79] and two studies in transgenic mice that overexpress mutant amyloid precursor protein (APP) via a doxycycline-regulated promoter [80, 81] show that subchronic or chronic suppression of Aβ production arrested Aβ plaque formation but did not result in observable clearance of existing plaques. In a related study [82], Aβ(1-42) immunization of Tg2576 mice prior to significant Aβ deposition, at an age with modest deposition or at an age with significant deposition, showed that immunization prevented additional Aβ deposition but did not result in significant clearance of pre-existing Aβ. These studies indicate that mature, dense-core Aβ plaques are not in significant equilibrium with soluble Aβ pools. Studies in transgenic mouse models of AD reporting decreased soluble Aβ oligomer levels and reduced cognitive deficits with increasing Aβ plaque levels also provide support for the concept that Aβ plaques may be a sink for soluble Aβ species [83, 84].
However, in an elegant study in APP transgenic mice using a novel microdialysis technique, the half-life of low-molecular-weight Aβ species in hippocampal interstitial fluid following inhibition of Aβ production by a potent secretase inhibitor was doubled in 12- to 15-month-old mice with Aβ deposits compared with 3-month-old mice without Aβ deposits [85]. On the basis of these results, it was suggested that insoluble Aβ was in equilibrium with soluble Aβ in the interstitial fluid. In a related study using similar techniques, the temporal changes of low-molecular-weight Aβ species in interstitial fluid and Aβ levels in Tris-buffered saline (TBS), SDS, and formic acid extracts of brain tissues of 3-, 12-, and 24-month-old APP transgenic mice were reported [86]. A significant age-dependent decrease in low-molecular-weight Aβ in interstitial fluid and significant increases in Aβ species in SDS and formic acid extracts of brain tissues were found. Although Aβ increased approximately seven-fold from baseline in the TBS extract of brain tissues, the level of Aβ in TBS extracts was less than 2% of the total Aβ in SDS and formic acid extracts. These results indicated an age-dependent sequestration of Aβ as non-diffusible cell matrix and membrane-bound Aβ and deposited Aβ plaques. Acute γ-secretase inhibition of Aβ production in plaque-free and plaque-rich mice suggested that Aβ(1-42) in the interstitial fluid of plaque-rich mice was derived primarily from Aβ(1-42) sequestered in brain parenchyma rather than from new biosynthesis. However, it was not possible to determine whether cell matrix and membrane-bound Aβ or Aβ plaques or both forms of Aβ were the source of Aβ(1-42) in the interstitial fluid of aged mice.
In a more recent study of the temporal changes of Aβ species in the interstitial fluid and brain tissues of APP transgenic mice, Takeda and colleagues [87], using a 1,000-kDa-molecular-weight cutoff microdialysis probe, reported the temporal changes in soluble Aβ oligomer levels. In that study, consistent with previous studies [85, 86], a significant, age-dependent increase in Aβ levels in TBS and formic acid extracts of brain tissues was found, and TBS extractable Aβ was less than 1% of formic acid extractable Aβ. However, unlike previous studies using a 35-kDa-molecular-weight cutoff microdialysis probe [85, 86], a significant, age-dependent increase in interstitial fluid Aβ levels was found by using the larger-pore-sized microdialysis probe. The majority of the Aβ in interstitial fluid was determined to be higher-molecular-weight soluble Aβ oligomers, which showed an age-dependent increase relative to lower-molecular-weight Aβ species. Temporal changes in soluble Aβ oligomer levels in interstitial fluid and TBS brain extracts showed a significant positive correlation with formic acid Aβ extract levels. Comparison of high- and low-molecular-weight interstitial fluid Aβ levels at baseline and following acute treatment with a γ-secretase inhibitor showed slower clearance of higher-molecular-weight Aβ oligomers compared with low-molecular-weight Aβ species.
Narayan and colleagues [88] have recently used single-molecule imaging techniques to investigate interactions between Aβ peptides and hippocampal cell membranes and reported results indicating that Aβ oligomers preferentially interact with membranes compared with Aβ monomer, thereby providing support for the results observed in the microdialysis studies. Thus, this study, coupled with those of the temporal changes of Aβ species in APP transgenic mice [85–87], does not resolve the controversy regarding the sink/source relationship between fibrillar and soluble Aβ species.
The sink/source relationship between soluble and insoluble Aβ pools is complex, not fully understood, and subject to ongoing debate. Two different forms of Aβ plaques are present in the AD brain: vascular amyloid plaques that are primarily composed of Aβ(1-40) and Aβ plaques that are primarily composed of Aβ(1-42) [89]. In vitro studies have shown that fibrillar Aβ(1-40) and Aβ(1-42) are in equilibrium with soluble Aβ [90, 91] but that recycling of Aβ(1-40) fibrils is significantly faster that Aβ(1-42) fibrils [91]. Moreover, it has been reported that plaque deposition proceeds in two distinct kinetic phases: an initial, reversible deposition phase followed by a time-dependent irreversible deposition phase [92]. Furthermore, a recent study of the rates of formation of Aβ oligomers and fibrils provided evidence that the formation of soluble Aβ oligomers from monomeric Aβ is catalyzed by fibrillar Aβ [73]. This study not only indicated a mechanistic linkage between soluble Aβ oligomers and fibrillar Aβ but also provided evidence showing that fibrillar Aβ can be a ‘source’ of soluble Aβ via catalyzed oligomerization of Aβ monomer rather than via a disaggregation process. This study also provided an alternative explanation for the study by Koffie and colleagues [93], who reported a halo of soluble Aβ oligomers surrounding Aβ plaques and proposed that Aβ plaques were a possible source of soluble Aβ oligomers.
Although precise details remain to be fully elucidated, collectively, over two decades of studies on the mechanisms of formation of oligomeric and fibrillar Aβ, the temporal distribution of Aβ species in vitro and in vivo, the age-dependent effect of Aβ immunization, and the effects of subchronic and chronic suppression of Aβ production upon in vivo Aβ plaque levels show a significant, age-dependent increase in brain levels of soluble Aβ oligomers and deposited fibrillar Aβ. Collectively, the studies suggest that mature, dense-core Aβ plaques are not in equilibrium to any significant extent with soluble pools of Aβ, but that Aβ sequestered in the cell matrix and membranes and immature plaques is in equilibrium with soluble Aβ pools, and that fibrillar Aβ catalyzes Aβ monomer oligomerization, giving rise to soluble Aβ oligomers and growth of Aβ plaques.
Neuronal toxicity of amyloid-beta species
Monomeric Aβ, primarily the Aβ(1-40) and Aβ(1-42) peptides, is produced in various cell types throughout the body and reported to have trophic properties in vitro[94, 95]. There are no reports suggesting that monomeric Aβ possesses any direct cellular toxicity at physiologically relevant concentrations. Insoluble fibrillar aggregates of Aβ, vascular amyloid and Aβ plaques, exhibit relatively low in vitro toxicity and have been proposed to be an in vivo mechanism for removal of the more toxic soluble Aβ species [83, 96, 97]. It was first suggested in 1995 that soluble Aβ species rather than fibrillar plaques could trigger neurotoxicity leading to AD [98], and in the subsequent decades, many studies have shown soluble Aβ oligomers to be the most toxic Aβ form, causing both acute synaptotoxicity and inducing neurodegenerative processes [5–10, 99–102].
Low-picomolar levels of soluble Aβ oligomers have been reported to have trophic properties in vitro[103–105], suggesting that therapeutic targeting of soluble Aβ oligomers may need to modulate oligomer levels versus completely sequestering or preventing formation of soluble Aβ oligomers. However, the concentration of Aβ42 at which enhancement of long-term potentiation (LTP) was observed in these studies was one to two orders of magnitude greater than levels of soluble Aβ oligomers reported in the cerebrospinal fluid (CSF) of human patients with AD, and the concentration above which inhibition of LTP was observed was an order of magnitude greater than the total levels of soluble Aβ species reported in the CSF of human patients with AD [65]. Thus, the relevance of the reported in vitro trophic properties of soluble Aβ oligomers to in vivo conditions remains to be established.
Soluble Aβ oligomers bind with high affinity to synapses on a subset of hippocampal and cortical neurons [19, 40, 106–108], indicative of specific binding to discrete cell surface receptors. In rodent hippocampal slice preparations, synaptic binding leads to rapid inhibition of LTP [19, 40, 109], and injection of various soluble Aβ oligomer preparations directly into the rodent brain leads to reversible impairment of cognitive function [31, 33, 110]. This aberrant signaling also causes accumulated biochemical damage within neurons [100, 111, 112], such as hyperphosphorylation of tau [100, 111–113], suggesting a linkage between Aβ and tau pathologies [114–116]. Soluble Aβ oligomers have been isolated from extracts of postmortem AD brain tissue and from transgenic AD animal models [37, 52, 117–119] and have been reported to be elevated in human AD brain relative to non-demented older patients [59, 61, 62, 65, 119–124]. Importantly, a recent study suggests a correlation between CSF levels of soluble Aβ oligomers and cognitive deficits in human patients with AD [61, 65]. These findings support the view that soluble Aβ oligomers interfere acutely with normal synaptic functions and contribute significantly to the memory loss and cognitive dysfunction characteristic of AD.
Structure and activity of soluble amyloid-beta oligomer species
There is substantial ongoing debate and research concerning the structure and activity of soluble Aβ species [5, 7–9, 33, 41, 52, 72, 99, 125–127]. Various soluble Aβ oligomer species have been reported to display synaptic toxicity or induce cognitive deficits, including dimers [33, 35, 128, 129], trimers [32], dodecamers [33, 37, 40], and larger soluble Aβ oligomers with molecular weights of 90 to 650 kDa (20 to 150 mers) [19, 130, 131]. Unfortunately, the different methodologies for the preparation, characterization, and evaluation of soluble Aβ oligomer species by various research groups impede a direct comparison of the results reported, and few studies have directly compared the toxicities of different soluble Aβ species while using the same techniques.
Two studies have reported the comparative toxicities of different soluble Aβ oligomer species by using LDH (lactate dehydrogenase release) and MTT (oxidoreductase activity) cell viability assays. Deshpande and colleagues [45] examined the relative toxicities of purified spherical Aβ(1-42) oligomers [50], ADDLs [132], and fibrils [50]. However, because solutions of soluble Aβ oligomers in the neurobasal medium used in this study have been shown to change with time [19], it is not possible to draw definitive structure-activity conclusions from the results of this study. In the second study, Ono and colleagues [133] reported relative toxicities of purified, cross-linked Aβ(1-40) dimers, trimers, and tetramers, which were shown to be relatively stable under the assay conditions. In an MTT assay, half maximal effective concentration values were 67.3, 41.6, 24.5, 20.5, and 57.6 μM, respectively, for monomer, dimer, trimer, tetramer, and fibrils. Comparable toxicity was obtained in an LDH assay. The micromolar concentrations of Aβ species used in this study were approximately six orders of magnitude greater than in vivo Aβ concentrations, and the relevance of cell culture MTT-type assays to the in vivo synaptotoxicity of Aβ species has been questioned [134]. Therefore, the results of the study by Ono and colleagues provide little understanding of the relative in vivo toxicities of soluble Aβ species.
More recently, cognitive effects in vivo were assessed in rats by using the alternating lever cyclic ratio assay following intracerebroventricular (ICV) injections of cell- and synthetically derived soluble Aβ oligomers [33]. Monomer and low-n-mer soluble Aβ oligomers derived from 7PA2 cells [29, 135–137], trimer and a dodecameric species (Aβ*56) extracted from Tg2576 mouse brain [37], and synthetic soluble Aβ oligomers (ADDLs) [43] were compared in this study. Injection of conditioned media from 7PA2 cells caused significant cognitive deficits. Evaluation of size exclusion chromatography (SEC)-enriched dimer or trimer fractions of 7PA2 cell-conditioned media showed significant cognitive deficits following injection of dimer-enriched fractions but a non-significant effect upon injection of trimer-enriched fractions. SEC-purified monomer had no effect. Aβ monomer was the predominant Aβ species in unfractionated 7PA2 conditioned media, and the amounts of dimer and trimer injected in the dimer- and trimer-enriched fractions were considerably greater than the amounts injected in unfractionated conditioned media. However, cognitive effects following injection of unfractionated conditioned media were comparable to or exceeded the observed effects following treatment with dimer- and trimer-enriched fractions. Thus, there is an inherent ambiguity regarding the results reported for 7PA2-derived Aβ species that is difficult to explain. One possible explanation is that a higher-order soluble Aβ oligomer species contributes to the cognitive deficits observed upon injection of unfractionated 7PA2 conditioned media and is a more potent inhibitor of cognitive function than Aβ dimer or trimer species or both. Another possible explanation is that combinations of different soluble Aβ oligomer species, perhaps interacting differently with different neuronal receptors, have an additive or synergistic toxicological effect. Trimers extracted from aged Tg2576 mouse brain also failed to elicit significant cognitive deficits. However, consistent with other in vivo efficacy studies [37], a dodecameric soluble Aβ oligomer extracted from aged Tg2576 mouse brain (Aβ*56) caused significant cognitive deficits. Synthetic soluble Aβ oligomers (ADDLs) also caused cognitive deficits following ICV injection. The results of this study show that ICV injection of soluble Aβ oligomers from different sources causes cognitive deficits in wild-type rats and that these deficits are reversible. The results of the study show that soluble Aβ oligomer containing conditioned media of 7PA2 cells is more potent than solutions of Aβ*56 obtained from aged Tg2576 mouse brains or solutions of synthetically prepared soluble Aβ oligomers (ADDLs). However, because it is not possible to quantitatively characterize the exact nature or distribution of soluble Aβ species in these different preparations, it is not possible to draw definitive conclusions from this study regarding the structure-activity relationships between individual soluble Aβ oligomer species.
In a more recent study, Moreth and colleagues [19] prepared, characterized, and evaluated the hippocampal binding and effects on neurotransmission of spheroidal, protofibrillar, and fibrillar Aβ aggregates. Under the conditions used to test for hippocampal binding and neurotransmission, the different species where shown to be relatively unchanged. Monomeric and fibrillar Aβ did not bind to mature hippocampal neurons (DIV21) or effect neurotransmission at concentrations as high as 1 μM. In contrast, spheroidal and protofibrillar Aβ aggregates displayed punctate binding to mature hippocampal neurons and impaired neurotransmission with nanomolar potency. Significantly, the mode of impairment of neurotransmission was different for spheroidal Aβ aggregates, which impaired LTP at 30 nM, compared with protofibrillar aggregates, which impaired basal neurotransmission at 100 nM. Spheroidal Aβ aggregates had no effect on basal neurotransmission at concentrations as high as 100 nM. Although this study was unable to address the comparative neurotoxicity of discrete soluble Aβ oligomers, it did show that different forms of soluble Aβ oligomers can trigger distinct neuronal activities.
At this point, the exact structures of the toxicologically relevant soluble Aβ oligomer species have not been determined to the complete exclusion of other possible structures, and analytical tools do not exist to characterize the Aβ oligomers that form at concentrations in the AD brain [44, 138]. The numerous studies reporting neuronal toxicity for different soluble Aβ oligomers support the conclusion that multiple soluble Aβ oligomer species exhibit neuronal toxicity, rather than a single, discrete toxic species. This suggestion of multiple toxic soluble Aβ oligomer species may also explain the plethora of reported neuronal receptors that mediate the effects of soluble Aβ oligomers (Table 1) [139–165], a discussion of which is well beyond the scope of this review. (See the perspective of Benilova and De Strooper [166] for a good introduction to this complex and controversial field of study.)
Despite recognition that soluble Aβ oligomers are key structures causing AD memory malfunction and cognitive deficits, drug discovery efforts targeting these species have been hampered by perceived technical difficulties of generating physiologically relevant preparations of synthetic soluble Aβ oligomers and by differing terminologies and methodologies used by various researchers [167]. However, well-characterized and documented preparations of synthetic soluble Aβ oligomers have been reported by numerous researchers [19, 40, 43, 95, 100, 107, 108, 111, 168–170] that generate soluble Aβ oligomers with little or no detectable fibrillar Aβ species. With the availability of a number of well-documented preparations of different soluble Aβ oligomer species and tools for comparative characterization, it is hoped that additional side-by-side testing of various soluble Aβ oligomer preparations in different toxicity paradigms such as reversal of basal neurotransmission, LTP inhibition, changes in AMPA receptor trafficking, tau phosphorylation, and loss of dendritic spines [19, 108, 109, 170] will be conducted and reported.
Amyloid-beta immunotherapies in development
Successful immunotherapies developed for most diseases rely upon antibodies that possess high selectivity for a particular target antigen connected to the disease. However, all Aβ-directed immunotherapies currently in clinical development are based on non-selective antibodies that bind multiple Aβ species. Treatment with non-selective anti-Aβ antibodies that bind monomeric or oligomeric Aβ (or both) potentially could succeed in a prodromal treatment paradigm by reducing total brain Aβ to levels below the toxicological threshold. However, given the high concentrations of monomeric and fibrillar Aβ compared with soluble Aβ oligomers in the AD brain [57–65] and the low levels of antibodies that penetrate the brain from the periphery [171], it will be very challenging for non-selective anti-Aβ antibodies that bind monomeric or fibrillar Aβ (or both) to show efficacy when administered to patients with mild cognitive impairment or mild-to-moderate AD. In contrast, the acute synaptic toxicity and very low brain levels of soluble Aβ oligomers suggest that anti-Aβ antibodies with selective affinity for soluble Aβ oligomers could provide therapeutic benefit for patients with mild cognitive impairment or mild-to-moderate AD.
It might seem difficult to generate a monoclonal antibody that does not bind monomeric or fibrillar Aβ yet possesses high affinity for structurally heterogeneous soluble Aβ oligomers. However, monomeric, oligomeric, and fibrillar Aβ species have been reported to have distinct conformational characteristics [172–175]. Moreover, antibodies with selective affinity for soluble Aβ oligomers versus monomeric and fibrillar Aβ have been reported [9, 176, 177]. These several examples of antibodies suggest that therapeutically relevant soluble Aβ oligomer-directed antibodies are indeed feasible.
A number of recent publications have reviewed Aβ immunotherapies currently in clinical trials, with an emphasis on the efficacy of the drugs for treating AD [178–187], and the clinical results will not be presented in this review, with the exception of an analysis of the brain levels of solanezumab and bapineuzumab in recently reported clinical trials. Instead, this review will focus on a discussion of the comparative affinities of the Aβ antibodies in clinical development for monomeric, soluble oligomeric, and fibrillar Aβ species. Notably, none of the Aβ immunotherapies currently in clinical development selectively targets soluble Aβ oligomers, as discussed below and summarized in Table 2.
Solanezumab
Solanezumab is unique among Aβ antibodies currently in clinical development because it does not bind fibrillar Aβ. Solanezumab is a humanized, IgG1 monoclonal antibody derived from the murine monoclonal antibody, 266. 266 was selected for its high-affinity binding to soluble Aβ, although the exact nature of the soluble Aβ it binds was not reported. It has been suggested that 266 is a conformation-specific antibody that solely recognizes soluble Aβ and readily binds monomeric Aβ [188–190], without binding APP or the C-terminal APP cleavage product of α-secretase [189]. It has been reported that 266 selectively sequesters Aβ monomer and dimer in the periphery of 3-month-old APP transgenic mice [190], and definitive evidence for 266 binding of monomeric Aβ has been reported [191]. A study using crossed-linked soluble Aβ oligomers showed 266 could immunoprecipate Aβ monomer and low-molecular-weight dimer, trimer, and possibly tetramer [192]. A study involving a competitive ELISA showed that 266 had a seven-fold higher affinity for monomeric Aβ than synthetic soluble Aβ oligomers [193]. Several studies have shown that 266 does not bind Aβ plaques or vascular amyloid [188, 190, 194]. Thus, according to the available data, solanezumab and 266 bind monomeric and lower-molecular-weight soluble Aβ oligomer species, with a preference for monomeric Aβ, but do not bind fibrillar Aβ species.
Clinical trial pharmacokinetic data for brain levels of solanezumab have not been reported. However, target engagement data reported for the phase 2, multiple ascending-dose clinical trials (Table 3) [183] provide a possible understanding of the lack of efficacy of solanezumab in clinical trials [186]. The data for the 400 mg/month and 400 mg/week dose levels show approximately a 2.7-fold increase in solanezumab bound Aβ at the higher dose level (5,858 versus 15,825 pg/mL total Aβ40 and Aβ42) [183], showing that there was incomplete target engagement at the 400 mg/month dose level used in the pivotal phase 3 trials. Solanezumab binds Aβ monomer with higher affinity than soluble Aβ oligomers and does not bind or disaggregate fibrillar Aβ. Because incomplete target engagement occurred at the dose level used in the phase 3 trials, it is unlikely any solanezumab was available to bind soluble Aβ oligomers; thus, the lack of efficacy of solanezumab at the dose levels used in the phase 3 clinical trials is not unexpected.
Bapineuzumab
Bapineuzumab is a humanized, IgG1 monoclonal antibody derived from the murine antibody, 3D6. Bapineuzumab and 3D6 are non-selective Aβ antibodies that bind with high affinity to monomeric Aβ [192], soluble Aβ oligomers [170], and fibrillar Aβ [195–197] but do not recognize APP or the product of β-secretase cleavage of APP [198]. In a competitive ELISA assay study, 3D6 was found to bind monomeric Aβ and synthetic soluble Aβ oligomers with similar affinity [193]. Babineuzumab/3D6 bind vascular amyloid and Aβ plaques in hAPP transgenic mice brains and human AD brains and clear vascular amyloid and Aβ plaques in hAPP transgenic mice brains [188, 197, 199–201]. Thus, bapineuzumab and 3D6 do not distinguish between the various Aβ species in the AD brain.
Mean CSF levels of bapineuzumab reported in the phase 2, multiple ascending-dose pharmacokinetic clinical studies were 4.9, 18.1, 27.2, and 44.7 ng/mL, respectively, at dose levels of 0.15, 0.5, 1.0, and 2 mg/kg (six infusions, 13 weeks apart) [202], which upon conversion to molar concentrations are 0.03, 0.12, 0.18, and 0.3 pmol/mL, respectively, based on a bapineuzumab molecular weight of 150 kDa. In the same studies, placebo-treated mean CSF levels of Aβ(1-42), Aβ(x-42), and Aβ(1-40) were 376.7, 537.9, and 6,705.4 pg/mL, respectively, which upon conversion to molar concentrations are 0.08, 0.12, and 1.55 pmol/mL, respectively (assuming the molecular weight of Aβ(x-42) is the same as that of Aβ(1-40)). The highest bapineuzumab dose level in phase 3 pivotal trials was 1 mg/kg (six infusions, once every 13 weeks) [187]. Thus, the total mean Aβ CSF levels were approximately an order of magnitude greater than mean levels of bapineuzumab. Because bapineuzumab binds monomeric Aβ with similar affinity to soluble Aβ oligomers and fibrillar Aβ and because Aβ soluble oligomer levels are orders of magnitude less than Aβ monomer and fibrils levels in the AD brain [58–65], it is clear that bapineuzumab was not dosed high enough in the pivotal phase 3 trials to effectively sequester soluble Aβ oligomers. Thus, the absence of efficacy of bapineuzumab in clinical trials is not unexpected.
Crenezumab
Crenezumab (MABT5102A or MABT) is a humanized, IgG4 monoclonal anti-Aβ antibody that was engineered to reduce Fcγ receptor-mediated microglia activation and minimize adverse effects due to vasogenic edema and cerebral microhemorrhage [203, 204]. Crenezumab binds Aβ monomer, soluble Aβ oligomers, and fibrillar Aβ with similar affinities and binds plaques in hAPP transgenic mice and human AD brain tissues [203, 204]. Similar binding of crenezumab to human Aβ(1-40) and Aβ(1-42) peptides and mouse/rat Aβ(1-42) has also been reported [204]. These results indicate that crenezumab does not possess selectivity for different Aβ species [204]. Consistent with a lack of selectivity, crenezumab prevents Aβ aggregation and disaggregates pre-aggregated Aβ species. Thus, crenezumab is similar to bapineuzumab with respect to non-selective binding of monomeric, oligomeric, and fibrillar Aβ species.
Ponezumab
Ponezumab is a humanized, modified IgG2 monoclonal antibody derived from the murine monoclonal antibody, 2H6. This progenitor murine antibody binds to monomeric Aβ(1-40) with high affinity but does not bind to Aβ(1-16), Aβ(1-28), Aβ(1-38), Aβ(1-42), or Aβ(1-43) [205]. Intracranial injection of 2H6 and its deglycosylated form into plaque-rich, 20-month-old Tg2576 mice followed by histological analysis of total Aβ load in the hippocampus and frontal cortex showed significant plaque reduction in both regions compared with control tissues [206], suggesting that these antibodies bind and disaggregate fibrillar amyloid. More recent studies show that ponezumab labels amyloid plaques in 14-month-old Tg2576 mouse and human AD brains [207], demonstrating binding to fibrillar Aβ. Binding of ponezumab to Tg2576 mouse brain plaque was comparable to the known high-affinity binding of the N-terminal Aβ antibody 6E10. Ponezumab was also reported to bind monomeric, oligomeric, and fibrillar forms of Aβ(1-40) but did not show appreciable binding to monomeric, oligomeric, or fibrillar forms of Aβ(1-42) [207]. Thus, although ponezumab displays selectivity for Aβ(1-40) versus other Aβ peptide isoforms, it is relatively non-selective for monomeric, soluble oligomeric, or fibrillar Aβ species.
BiiB037
BiiB037 is a human IgG1 monoclonal antibody derived from a patient with AD by using reverse translational medicine methodology [208]. In vitro BiiB037 binds fibrillar Aβ(1-42) with high affinity but does not bind soluble Aβ(1-40) [209]. Although explicit details of the binding characteristics of BiiB037 have not been publicly disclosed, results reported by Dunstan and colleagues [209] and data in the patent covering BiiB037 [208] suggest that BiiB037 is very similar, or identical, to antibody NI-101.11, which binds with high affinity to Aβ plaques in human AD brain tissue samples [208]. Such binding is not blocked by monomeric Aβ(1-16) or Aβ(1-42), showing selective affinity for fibrillar Aβ versus Aβ monomers [208]. Comparison of binding affinity in an Aβ plate-based ELISA showed greater than 100-fold-higher affinity for fibrillar Aβ(1-42) than for monomeric Aβ(1-42) [208]. SEC studies showed that NI-101.11 bound to fluorescein isothiocyanate-labeled Aβ(1-42) in the early-eluting peak (non-retained, large Aβ species) but that little binding was associated with the retained, low-molecular-weight Aβ peak [208]. Many researchers have deployed SEC to separate higher-molecular-weight soluble Aβ oligomer species from low-molecular-weight Aβ species [20, 44, 130, 155, 210–212], and in all cases the higher-molecular-weight, early-eluting peak contains soluble Aβ oligomers or Aβ protofibrils or both. Together these results show that NI-101.11 binds soluble Aβ oligomers and protofibrils in addition to fibrillar Aβ. Consistent with this conclusion is the finding that NI-101.11 inhibits the formation of Aβ fibrils in vitro[208]. Thus, according to the available data, BiiB037/NI-101.11 binds soluble Aβ oligomers and fibrillar Aβ with high affinity and binds monomeric Aβ with low affinity.
BAN2401
BAN2401 is a humanized, IgG2a monoclonal antibody derived from the murine precursor mAb158, which was raised against Aβ protofibrils generated from Aβ(1-42)-E22G, the arctic mutant of the Aβ peptide [210]. Although mAb158 is claimed to be a ‘highly protofibril-selective monoclonal antibody’ [213], published data show that it binds fibrillar Aβ with a high affinity similar to that of protofibrils in a dot-blot assay [210, 214] and binds fibrillar deposits in transgenic APP-ArcSwe mice brain (see Figure S2 in [213]). In a more recent study, mAb158 was shown to bind soluble Aβ oligomer fractions in the molecular weight range of 80 to 2,000 kDa under conditions in which protofibrillar species were not detected [119]. In this study, mAb158 showed essentially no binding to low-molecular-weight Aβ oligomeric species in the molecular weight range of 5 to 80 kDa. A lack of binding to low-molecular-weight Aβ species - monomeric to tetrameric Aβ(1-40), Aβ(1-16), and Aβ(17-40) - was found in an earlier study [213]. Thus, mAb158 binds higher-molecular-weight Aβ aggregates, including soluble Aβ oligomers, protofibrils, and fibrillar Aβ. Consistent with the binding of mAb158 to fibrillar Aβ, intracranial injection of mAβ158 in a transgenic APP-Swe mouse model was reported to cause a significant reduction in plaque burden by 72 hours after injection [214]. In a subsequent study in a transgenic APP-ArcSwe mouse model [213], 4 months of weekly intraperitoneal injections of mAb158 to 9- to 10-month-old mice with modest plaque burden resulted in a significant reduction of Aβ protofibrils (74%) but no significant reduction in total soluble or insoluble Aβ levels in the cerebral cortex or hippocampus as determined by immunohistochemical analysis. The differences in effects of mAb158 on existing plaque burden in these two studies may be due to different administration routes (direct intracranial versus peripheral injections). As noted by Lord and colleagues [213], it may also be attributable to the APP-ArcSwe mouse model used in the more recent study, a model that exhibits more highly insoluble dense-core plaques, preventable only by early and continuous Aβ immunotherapy. Thus, mAb158/BAN2401 binds higher-molecular-weight soluble Aβ oligomers (referred to as protofibrils in the mAb158 literature) and fibrillar Aβ with high affinity and has low affinity for binding monomeric Aβ and lower-molecular-weight soluble Aβ oligomers.
Gantenerumab
Gantenerumab is a humanized monoclonal antibody that was optimized to have high-affinity binding of fibrillar Aβ and shows high-affinity binding to diffuse and dense-core plaques in human AD brain and APP transgenic mouse brain tissues [215]. Gantenerumab also binds soluble Aβ oligomers with high affinity and, to a lesser extent, Aβ monomer. The reported binding constants (Kds) for gantenerumab binding to fibrillar Aβ, soluble Aβ oligomers, and Aβ monomer are 0.6, 1.2, and 17 nM, respectively [215]. The dissociation constants (kds) for gantenerumab-Aβ complexes were reported to be 2.8 × 10-4, 4.9 × 10-4, and 1.2 × 10-2, respectively, for fibrillar Aβ, Aβ oligomers, and Aβ monomer [215], suggesting a more rapid exchange of antibody-monomer complex compared with antibody-fibril or oligomer complexes. The selective affinity of gantenerumab for fibrillar Aβ and soluble Aβ oligomers suggests that it may be similar to BiiB013/NI-101.11 and BAN2401/mAb158. Consistent with its high-affinity binding to fibrillar Aβ, gantenerumab caused a significant, dose-dependent reduction in amyloid plaques in human AD brain tissue [215]. Immunohistochemical analysis of PS2APP transgenic mice brain tissue 3 days after intravenous injection of gantenerumab showed dose-dependent binding to amyloid plaques. Significantly, pharmacokinetic analysis in PS2APP transgenic mice following a single intravenous bolus dose showed sustained binding of gantenerumab to plaques in the brain for up to 9 weeks post-dosing, even though there was no detectable antibody in the plasma beyond 3 weeks post-dosing [215]. In a chronic treatment study in 5- to 6-month-old PS2APP transgenic mice, gantenerumab caused a significant reduction in pre-existing small plaques; however, there was an increase in larger plaques [215]. These results suggest that gantenerumab causes a redistribution of Aβ from small plaques to large plaques, possibly via disaggregation of smaller, less mature plaques. Gantenerumab-mediated disaggregation of existing plaques is consistent with the observations of vasogenic edema and microhemorrhage in human patients with AD treated with gantenerumab in clinical trials [216]. Thus, gantenerumab non-selectively binds soluble Aβ oligomers and fibrillar Aβ with similar high affinity in comparison with its lower-affinity binding of monomeric Aβ.
SAR228810
SAR228810 is a humanized, monoclonal antibody derived from the murine monoclonal antibody 13C3, which was raised to Aβ protofibrils derived from Aβ(1-42) [217, 218]. The humanized antibody was engineered into an IgG4 framework to reduce binding to Fcγ and C1q receptors and decrease risks of amyloid-related imaging abnormalities [219]. Although specific binding characteristics of SAR228810 have not been publicly disclosed, results reported by Pradier and colleagues [219] and data in the patent application claiming humanized variants of 13C3 [220] suggest that SAR228810 is very similar, or identical, to antibody LP09027 [220]. Antibody 13C3 and its humanized variants are reported to bind with higher affinity to SEC-separated protofibrillar Aβ(1-42) than to low-molecular-weight Aβ(1-42) [217, 218, 220]. As noted previously, many researchers have used SEC to separate higher-molecular-weight soluble Aβ oligomer species from low-molecular-weight Aβ species [20, 44, 130, 155, 210–212], and in all cases the higher-molecular-weight, early-eluting peak contains soluble Aβ oligomers or Aβ protofibrils or both. Moreover, as reported by Hepler and colleagues [44], the lower-molecular-weight, late-eluting peak contains predominately monomeric Aβ. Thus, these data show that 13C3 and its humanized variants selectively bind Aβ oligomers or protofibrils (or both) compared with monomeric Aβ. In competitive ELISA experiments, monomeric Aβ(1-28), Aβ(1-16), or Aβ(25-35) does not prevent binding of humanized 13C3 to plates coated with Aβ(1-42) [220]. Ravetch and Fukuyama [218] reported that 13C3 binds only to the higher-molecular-weight Aβ species contained in the supernatants from 7PA2 cells, in contrast to the non-selective Aβ antibody 4G8 that binds low-, medium-, and higher-molecular-weight Aβ species. Collectively, these data show the selectivity of 13C3 for higher-molecular-weight Aβ oligomers versus lower-molecular-weight Aβ oligomers and monomer. Antibody 13C3 was shown to bind fibrillar Aβ(1-42) and label Aβ deposits in human AD brain tissue samples [218]. Antibody LP09027 was shown to bind fibrillar Aβ in vitro and to label AB deposits in brain sections from APP-PS1 mice [220]. Thus, the reported data suggest that 13C3/SAR228810 is very similar to mAb158/BAN2401 and binds higher-molecular-weight soluble Aβ oligomers (referred to as protofibrils in the 13C3/SAR228810 literature) and fibrillar Aβ with high affinity and has low affinity for binding monomeric Aβ and lower-molecular-weight soluble Aβ oligomers.
Conclusions
The lack of efficacy observed for Aβ immunotherapies in clinical development is not unexpected given their lack of selectivity for soluble Aβ oligomers compared with monomeric or fibrillar Aβ and given the tremendous quantities of monomeric or fibrillar Aβ in the AD brain relative to soluble Aβ oligomers. Aβ antibodies optimized to bind soluble Aβ oligomers selectively are much more likely to succeed in AD clinical trials and should be aggressively pursued.
The prediction that immunotherapies targeting soluble Aβ oligomers will elicit clinical benefit is supported by studies of human Aβ autoantibodies, of which only a subset appears to be disease-protective (in particular, the subset that preferentially recognizes Aβ oligomers) [221–223]. Thus, immunotherapeutics with high selectivity for soluble Aβ oligomers, which resemble these protective auto-antibodies, are expected to deliver a clinical advantage compared with the non-selective immunotherapies in clinical development.
Studies have demonstrated that antibodies with selective affinity for soluble Aβ oligomers can block soluble Aβ oligomer-mediated synaptotoxicity in cell cultures [108, 224] and rapidly normalize memory deficits in transgenic AD mouse models [176]. These findings support the concept that antibodies with selective affinity for soluble Aβ oligomers may be a more effective therapeutic strategy than antibodies with high affinity for monomeric or fibrillar Aβ or both.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADDL:
-
Amyloid-derived diffusible ligand
- APP:
-
Amyloid precursor protein
- Aβ:
-
Amyloid-beta
- CSF:
-
Cerebrospinal fluid
- ELISA:
-
Enzyme-linked immunosorbent assay
- ICV:
-
Intracerebroventricular
- LDH:
-
Lactate dehydrogenase release
- LTP:
-
Long-term potentiation
- SDS:
-
Sodium dodecyl sulfate
- SEC:
-
Size exclusion chromatography
- TBS:
-
Tris-buffered saline.
References
Alzheimer’s Disease International, World Alzheimer Report, 2010: The Global Economic Impact of Dementia. Alzheimer’s Disease International. 2010, [http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf]
Bleiler TW: 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013, 9: 208-245. [http://www.alz.org/downloads/facts_figures_2013.pdf]
Selkoe DJ: Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J Alzheimers Dis. 2001, 3: 75-80.
Walsh DM, Selkoe DJ: Aβ oligomers - a decade of discovery. J Neurochem. 2007, 101: 1172-1184. 10.1111/j.1471-4159.2006.04426.x.
Roychaudhuri R, Yang M, Hoshi MM, Teplow DB: Amyloid β-protein assembly and Alzheimer disease. J Biol Chem. 2009, 284: 4749-4753. 10.1074/jbc.R800036200.
Ondrejcak T, Klyubin I, Hu N-W, Barry AE, Cullen WK, Rowan MJ: Alzheimer’s disease amyloid β-protein and synaptic function. Neuromol Med. 2010, 12: 13-26. 10.1007/s12017-009-8091-0.
Sakono M, Zako T: Amyloid oligomers: formation and toxicity of Aβ oligomers. FEBS J. 2010, 277: 1348-1358. 10.1111/j.1742-4658.2010.07568.x.
Ferreira ST, Klein WL: The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem. 2011, 96: 529-543. 10.1016/j.nlm.2011.08.003.
Benilova I, Karran E, De Strooper B: The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012, 15: 349-357. 10.1038/nn.3028.
Hayden EY, Teplow DB: Amyloid β-protein oligomers and Alzheimer’s disease. Alzheimers Res Ther. 2013, 5: 60-10.1186/alzrt226.
Wong CW, Quaranta V, Glenner GG: Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci USA. 1985, 82: 8729-8732. 10.1073/pnas.82.24.8729.
Hardy JA, Higgins GA: Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992, 256: 184-185. 10.1126/science.1566067.
Klein WL, Krafft GA, Finch CE: Targeting small Aβ oligomers: the solution to an Alzheimer’s disease conundrum?. Trends Neurosci. 2001, 24: 219-224. 10.1016/S0166-2236(00)01749-5.
De Felice FG, Ferreira ST: β-Amyloid production, aggregation, and clearance as targets for therapy in Alzheimer’s disease. Cell Mol Neurobiol. 2002, 22: 545-563. 10.1023/A:1021832302524.
Citron M: Strategies for disease modification in Alzheimer’s disease. Nat Rev Neurosci. 2004, 5: 677-685. 10.1038/nrn1495.
Haas C: Initiation and propagation of neurodegeneration. Nat Med. 2010, 16: 1201-1204. 10.1038/nm.2223.
Broersen K, Rousseau F, Schymkowitz J: The culprit behind amyloid beta peptide related neurotoxicity in Alzheimer’s disease: oligomer size or conformation. Alzheimers Res Ther. 2010, 2: 12-10.1186/alzrt36.
Wilcox KC, Lacor RN, Pitt J, Klein WL: Aβ oligomer-induced synapse degeneration in Alzheimer’s disease. Cell Mol Neurobiol. 2011, 31: 939-948. 10.1007/s10571-011-9691-4.
Moreth J, Kroker KS, Schwanzar D, Schnack C, von Arnim CAF, Hengerer B, Rosenbrock H, Kussmaul L: Globular and protofibrillar Aβ aggregates impair neurotransmission by different mechanism. Biochem. 2013, 52: 1466-1476. 10.1021/bi3016444.
Walsh DM, Lamakin A, Benedek GB, Condron MM, Teplow DB: Amyloid β-protein fibrillogenesis: detection of a protofibrillar intermediated. J Biol Chem. 1997, 272: 22364-22372. 10.1074/jbc.272.35.22364.
Fezoui Y, Hartley DM, Harper JD, Khurana R, Walsh DM, Condron MM, Selkoe DJ, Lansbury PT, Finnk AL, Teplow DB: An improved method of preparing the amyloid β-protein for fibrillogenesis and neurotoxicity experiments. Amyloid. 2000, 7: 166-178. 10.3109/13506120009146831.
Lee S, Fernandez EJ, Good TA: Role of aggregation conditions in structure, stability, and toxicity of intermediates in the Aβ fibril formation pathway. Protein Sci. 2007, 16: 723-732. 10.1110/ps.062514807.
Paravastu AK, Qahwash I, Leapman RD, Meredith SC, Tycko R: Seeded growth of β-amyloid fibrils from Alzheimer’s brain-derived fibrils produces a distinct fibril structure. Proc Natl Acad Sci USA. 2009, 106: 7443-7448. 10.1073/pnas.0812033106.
Norlin N, Hellberg M, Filippov A, Sousa AA, Gröbner G, Leapman RD, Almqvist N, Antzutkin ON: Aggregation and fibril morphology of the Arctic mutation of Alzheimer’s Aβ peptide by CD, TEM, STEM and in situ AFM. J Struct Biol. 2012, 180: 174-189. 10.1016/j.jsb.2012.06.010.
Morgado I, Fändrich M: Assembly of Alzheimer’s Aβ peptide into nanostructured amyloid fibrils. Curr Opin Colloid Interface Sci. 2011, 16: 508-514. 10.1016/j.cocis.2011.06.016.
Tekirian TL, Yang AY, Glabe C, Geddes JW: Toxicity of pyroglutaminated amyloid β-peptides 3(pE)-40 and -42 is similar to that of Aβ1-40 and -42. J Neurochem. 1999, 73: 1584-1589.
Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball ML: β-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA. 1993, 90: 10836-10840. 10.1073/pnas.90.22.10836.
Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H: The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ. 2006, 2006: re1-
Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ: Aggregation of secreted amyloid β-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995, 270: 9564-9570. 10.1074/jbc.270.16.9564.
Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ: Amyloid-β oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002, 30: 552-557.
Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kushowski MA, Selkoe DJ, Ashe KH: Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005, 8: 79-84. 10.1038/nn1372.
Townsend M, Cleary JP, Mehta T, Hofmeister J, Lesne S, O’Hare E, Walsh DM, Selkoe DJ: Orally available compound prevents deficits in memory caused by the Alzheimer amyloid-β oligomers. Ann Neurol. 2006, 60: 668-676. 10.1002/ana.21051.
Reed MN, Hofmeister JJ, Jungbauer L, Welzel AT, Yu C, Sherman MA, Lesné S, LaDu MJ, Walsh DM, Ashe KH, Cleary JP: Cognitive effects of cell-derived and synthetically derived Aβ oligomers. Neurobiol Aging. 2011, 32: 1784-1794. 10.1016/j.neurobiolaging.2009.11.007.
Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM: Aβ oligomers inhibit synapse remodeling necessary for memory consolidation. Neurobiol Aging. 2011, 32: 2211-2218. 10.1016/j.neurobiolaging.2010.01.001.
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ: Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008, 14: 837-842. 10.1038/nm1782.
Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ: Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006, 572: 477-492. 10.1113/jphysiol.2005.103754.
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH: A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006, 440: 352-357. 10.1038/nature04533.
Chen Y-R, Clabe CG: Distinct early folding and aggregation properties of Alzheimer amyloid-β peptides Aβ40 and Aβ42. J Biol Chem. 2006, 281: 24414-24422. 10.1074/jbc.M602363200.
Bitan G, Vollers SS, Teplow DB: Elucidation of primary structure elements controlling early amyloid β-protein oligomerization. J Biol Chem. 2003, 278: 34882-34889. 10.1074/jbc.M300825200.
Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H: Globular amyloid β-peptide1-42 oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005, 95: 834-847. 10.1111/j.1471-4159.2005.03407.x.
Larson ME, Lesné SE: Soluble Aβ oligomer production and toxicity. J Neurochem. 2012, 120 (Suppl 1): 125-139.
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL: Diffusible, non-fibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998, 95: 6448-6453. 10.1073/pnas.95.11.6448.
Stine WB, Dahlgren KN, Krafft GA, LaDu MJ:In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J Mol Biol. 2003, 278: 11612-11622.
Hepler RW, Grimm KM, Nahas DD, Breese R, Dodson EC, Acton P, Keller PM, Yeager M, Wang H, Shughrue P, Kinney G, Joyce JG: Solution state characterization of amyloid β-derived diffusible ligands. Biochem. 2006, 45: 15157-15167. 10.1021/bi061850f.
Deshpande A, Mina E, Glabe C, Busciglio J: Different conformations of amyloid β induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006, 26: 6011-6018. 10.1523/JNEUROSCI.1189-06.2006.
Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG: Fibril specific, conformation depended antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegen. 2007, 2: 18-10.1186/1750-1326-2-18.
Rangachari V, Moore BD, Reed DK, Sonoda LK, Bridges AW, Conboy E, Hartigan D, Rosenberry TL: Amyloid-β(1-42) rapidly forms protofibrils and oligomers by distinct pathways in low concentrations of sodium dodecylsulfate. Biochem. 2007, 46: 12451-12462. 10.1021/bi701213s.
Gellermann GP, Byrnes H, Striebinger A, Ullrich K, Mueller R, Hillen H, Barghorn S: Aβ-globulomers are formed independently of the fibril pathway. Neurobiol Dis. 2008, 30: 212-220. 10.1016/j.nbd.2008.01.010.
Nimmrich V, Grimm C, Draguhn A, Barghorn S, Lehmann A, Schoemaker H, Hillen H, Gross G, Ebert U, Bruehl C: Amyloid β oligomers (Aβ1-42 globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J Neurosci. 2008, 28: 788-797. 10.1523/JNEUROSCI.4771-07.2008.
Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG: Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005, 280: 17294-17300. 10.1074/jbc.M500997200.
Hoshi M, Sato M, Matsumoto S, Noguchi A, Yasutake K, Yoshida N, Sato K: Spherical aggregates of β-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3β. Proc Natl Acad Sci USA. 2003, 100: 6370-6575. 10.1073/pnas.1237107100.
Noguchi A, Matsumura S, Dezawa M, Tada M, Yanazawa M, Ito A, Akioka M, Kikuchi S, Sato M, Ideno S, Noda M, Fukunari A, Muramatsu S-i, Itokazu Y, Sato K, Takahashi H, Teplow DB, Nabeshima Y-i, Kakita A, Imahori K, Hoshi M: Isolation and characterization of patient-derived, toxic, high mass amyloid β-protein (Aβ) assembly from Alzheimer disease brains. J Biol Chem. 2009, 284: 32895-32905. 10.1074/jbc.M109.000208.
Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin AL, Benedek GB, Selkoe DJ, Teplow DB: Amyloid β-protein fibrillogenesis: structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999, 274: 25945-25952. 10.1074/jbc.274.36.25945.
Harper JD, Wong SS, Lieber CM, Lansbury PT: Assembly of Aβ amyloid protofibrils: an in vitro model for a possible early event in Alzheimer’s disease. Biochem. 1999, 38: 8972-8980. 10.1021/bi9904149.
Lashuel HA, Hartley DM, Petre BM, Wall JS, Simon MN, Walz T, Lansbury PT: Mixtures of wild-type and a pathogenic (E22G) form of Aβ40 in vitro accumulate protofibrils, including amyloid pores. J Mol Biol. 2003, 332: 795-808. 10.1016/S0022-2836(03)00927-6.
Lasagna-Reeves CA, Glabe CG, Kayed R: Amyloid-β annular protofibrils evade fibrillar fate in Alzheimer disease brain. J Biol Chem. 2011, 286: 22122-22130. 10.1074/jbc.M111.236257.
Mehta PD, Pirttilä T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM: Plasma and cerebrospinal fluid levels of amyloid β proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000, 57: 100-105. 10.1001/archneur.57.1.100.
Delacourte A, Sergeant N, Champain D, Wattez A, Maurage C-A, Lebert F, Pasquier F, David J-P: Nonoverlapping but synergetic tau and APP pathologies in sporadic Alzheimer’s disease. Neurology. 2002, 59: 398-407. 10.1212/WNL.59.3.398.
Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, Allsop D, Nakagawa M: High-molecular-weight β-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010, 24: 2716-2726. 10.1096/fj.09-150359.
Karran E, Mercken M, De Stropper B: The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Disc. 2011, 10: 698-712. 10.1038/nrd3505.
Wolfe A, McCampbell A, Hatcher N, Tugusheva K, Haugabook S, Maxwell J, Wu G, Howell B, Renger J, Shughrue P, Savage M: A quantitative assay selective for amyloid oligomer species differentiates cerebrospinal fluid from Alzheimer’s disease and age-matched normal. Alzheimers Dementia. 2012, 8 (Supplement): P278-
Hölttä M, Hansson O, Andreasson U, Hertze J, Minthon L, Nägga K, Andreasen N, Zetterberg H, Blennow K: Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS One. 2013, 8: e66381-10.1371/journal.pone.0066381.
Lue L-F, Kuo Y-M, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J: Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999, 155: 853-862. 10.1016/S0002-9440(10)65184-X.
McDonald JM, Cairns NJ, Taylor-Reinwald L, Holtzman D, Walsh DM: The levels of water-soluble and triton-soluble Aβ are increased in Alzheimer’s disease brain. Brain Res. 2012, 1450: 138-147.
Savage MJ, Kalinina J, Wolfe A, Tugusheva K, Korn R, Cash-Mason T, Maxwell JW, Hatcher NG, Haugabook SJ, Wu G, Howell BJ, Renger JJ, Shughrue PJ, McCampbell A: A sensitive Aβ oligomer assay discriminates Alzheimer’s and aged control cerebrospinal fluid. J Neurosci. 2014, 34: 2884-2897. 10.1523/JNEUROSCI.1675-13.2014.
Johnson RD, Schauerte JA, Wisser KC, Gafni A, Steel DG: Direct observation of single amyloid-β(1-40) oligomers on live cells: binding and growth at physiological concentrations. PLoS One. 2011, 6: e23970-10.1371/journal.pone.0023970.
Johnson RD, Schauerte JA, Chang C-C, Wisser KC, Althaus JC, Carruthers CJL, Sutton MA, Steel DG, Gafni A: Single-molecule imaging reveals Aβ42:Aβ40 ratio-dependent oligomer growth on neuronal processes. Biophys J. 2013, 104: 894-903. 10.1016/j.bpj.2012.12.051.
Lansbury PT: Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci USA. 1999, 96: 3342-3344. 10.1073/pnas.96.7.3342.
Serpell LC: Alzheimer’s amyloid fibrils: structure and assembly. Biochim Biophys Acta. 2000, 1502: 16-30. 10.1016/S0925-4439(00)00029-6.
Blackley HK, Sanders GH, Davies MC, Roberts CJ, Tendler SJ, Wilkinson MJ: In-situ atomic force microscopy study of β-amyloid fibrillization. J Mol Biol. 2000, 298: 833-840. 10.1006/jmbi.2000.3711.
Kirkitadze MD, Condron MM, Teplow DB: Identification and characterization of key kinetic intermediates in amyloid β-protein fibrillogenesis. J Mol Biol. 2001, 312: 1103-1119. 10.1006/jmbi.2001.4970.
Caughey B, Lansbury PT: Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003, 26: 267-298. 10.1146/annurev.neuro.26.010302.081142.
Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TP: Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci USA. 2013, 110: 9758-9763. 10.1073/pnas.1218402110.
Jeong JS, Ansaloni A, Mezzenga R, Lashuel HA, Dietler G: Novel mechanistic insight into the molecular basis of amyloid polymorphism and secondary nucleation during amyloid formation. J Mol Biol. 2013, 425: 1765-1781. 10.1016/j.jmb.2013.02.005.
Stefani M: Biochemical and biophysical features of both oligomer/fibril and cell membrane in amyloid cytotoxicity. FEBS J. 2010, 277: 4602-4613. 10.1111/j.1742-4658.2010.07889.x.
Lanz TA, Himes CS, Pallante G, Adams L, Yamazaki S, Amore B, Merchant KM:The γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester reduces Aβ levels in vivo in plasma and cerebrospinal fluid in young (plaque-free) and aged (plaque-bearing) Tg2576 mice. J Pharmacol Exp Ther. 2003, 305: 864-871. 10.1124/jpet.102.048280.
Barten DM, Guss VL, Corsa JA, Loo A, Hansel SB, Zheng M, Munoz B, Srinivasan K, Wang B, Robertson BJ, Polson CT, Wang J, Roberts SB, Hendrick JP, Anderson JJ, Loy JK, Denton R, Verdoorn TA, Smith DW, Felsenstein KM: Dynamics of β-amyloid reductions in brain, cerebrospinal fluid, and plasma of β-amyloid precursor protein transgenic mice treated with a γ-secretase inhibitor. J Pharmacol Exp Ther. 2005, 312: 635-643.
Abramowski D, Wiederhold K-H, Furrer U, Jaton A-L, Neuenschwander A, Runser M-J, Danner S, Reichwald J, Ammaturo D, Staab D, Stoeckli M, Rueeger H, Neumann U, Staufenbiel M: Dynamics of Aβ turnover and deposition in different β-amyloid precursor protein transgenic mouse models following γ-secretase inhibition. J Pharmacol Exp Ther. 2008, 327: 411-424. 10.1124/jpet.108.140327.
Garcia-Alloza M, Subramanian M, Thyssen D, Borrelli LA, Fauq A, Das P, Golde TE, Hyman BT, Bacskai BJ: Existing plaques and neuritic abnormalities in APP:PS1 mice are not affected by administration of the gamma-secretase inhibitor LY-411575. Mol Neurodegen. 2009, 4: 19-10.1186/1750-1326-4-19.
Jankowsky JL, Slunt HH, Gonzales V, Savonenko AV, Wen JC, Jenkins NA, Copeland NG, Younkin LH, Lester HA, Younkin SG, Borchelt DR: Persistent amyloidosis following suppression of Aβ production in a transgenic model of Alzheimer disease. PLoS Med. 2005, 2: e355-10.1371/journal.pmed.0020355.
Melnikova T, Fromholt S, Kim HS, Lee D, Xu G, Price A, Moore BD, Golde TE, Felsenstein KM, Savonenko A, Borchelt DR: Reversible pathologic and cognitive phenotypes in an inducible model of Alzheimer-amyloidosis. J Neurosci. 2013, 33: 3765-3779. 10.1523/JNEUROSCI.4251-12.2013.
Das P, Murphy MP, Younkin LA, Younkin SG, Golde TE: Reduced effectiveness of Aβ1-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001, 22: 721-727. 10.1016/S0197-4580(01)00245-7.
Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puoliväli J, Lesné S, Ashe KH, Muchowski PJ, Mucke L: Accelerating amyloid-β fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007, 282: 23818-23828. 10.1074/jbc.M701078200.
Lesné S, Kotilinek L, Ashe KH: Plaque-bearing mice with reduced levels of oligomeric amyloid-β assemblies have intact memory function. Neurosci. 2008, 151: 745-749. 10.1016/j.neuroscience.2007.10.054.
Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM:In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003, 23: 8844-8853.
Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ: Dynamic analysis of amyloid β-protein in behaving mice reveals opposing changes in ISF vs. parenchymal Aβ during age-related plaque formation. J Neurosci. 2011, 31: 15861-15869. 10.1523/JNEUROSCI.3272-11.2011.
Takeda S, Hashimoto T, Roe AD, Hori Y, Spires-Jones TL, Hyman BT: Brain interstitial oligomeric amyloid β increases with age and is resistant to clearance from brain in a mouse model of Alzheimer’s disease. FASEB J. 2013, 27: 3239-3248. 10.1096/fj.13-229666.
Narayan P, Ganzinger KA, McColl J, Weimann L, Meehan S, Qamar S, Carver JA, Wilson MR, St George-Hyslop P, Dobson CM, Klenerman D: Single molecule characterization of the interactions between amyloid-β peptides and the membranes of hippocampal cells. J Am Chem Soc. 2013, 135: 1491-1498. 10.1021/ja3103567.
Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Younkin LH, Suzuki N, Younkin SG: Amyloid β protein (Aβ) in Alzheimer’s disease brain: biochemical and immunocytochemical analysis with antibodies specific for forms ending at Aβ40 or Aβ42(43). J Biol Chem. 1995, 270: 7013-1016. 10.1074/jbc.270.13.7013.
Wetzel R: Kinetics and thermodynamics of amyloid fibril assembly. Acc Chem Res. 2006, 39: 671-679. 10.1021/ar050069h.
Sánchez L, Madurga S, Pukala T, Vilaseca M, López-Iglesias C, Robinson CV, Giralt E, Carulla N: Aβ40 and Aβ42 amyloid fibrils exhibit distinct molecular recycling properties. J Am Chem Soc. 2011, 133: 6505-6508. 10.1021/ja1117123.
Esler WP, Stimson ER, Jennings JM, Vinters HV, Ghilardi JR, Lee JP, Mantyh PW, Maggio JE: Alzheimer’s disease amyloid propagation by a template-dependent dock-lock mechanism. Biochem. 2000, 39: 6288-6295. 10.1021/bi992933h.
Koffie RM, Mayer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL: Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009, 106: 4012-4017. 10.1073/pnas.0811698106.
Atwood CA, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, Martins RN: Amyloid-β: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-β. Brain Res Rev. 2003, 43: 1-16. 10.1016/S0165-0173(03)00174-7.
Giuffrida ML, Caraci F, Pagnataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A: β-Amyloid monomers are neuroprotective. J Neurosci. 2009, 29: 10582-10587. 10.1523/JNEUROSCI.1736-09.2009.
Baglioni S, Casamenti F, Bucciantini M, Luheshi LM, Taddei N, Chiti F, Dobson CM, Stefani M: Prefibrillar amyloid aggregates could be generic toxins in higher organisms. J Neurosci. 2006, 26: 8160-8162. 10.1523/JNEUROSCI.4809-05.2006.
Treusch S, Cyr DM, Lindquist S: Amyloid deposits: protection against toxic protein species?. Cell Cycle. 2009, 8: 1668-1674. 10.4161/cc.8.11.8503.
Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, Morgan TE, Rozovsky I, Stine WB, Snyder SW, Holzman TF, Krafft GA, Finch CE: Clusterin (apoJ) alters the aggregation of amyloid β-peptide (Aβ1-42) and forms slowly sedimenting Aβ complexes that cause oxidative stress. Exp Neurobiol. 1995, 136: 22-31. 10.1006/exnr.1995.1080.
Standridge JB: Vicious cycles within the neuropathophysiologic mechanisms of Alzheimer’s disease. Curr Alzheimers Res. 2006, 3: 95-107. 10.2174/156720506776383068.
Zemple H, Thies E, Mandelkow E, Mandelkow EM: Abeta oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010, 30: 11938-11950. 10.1523/JNEUROSCI.2357-10.2010.
Klyubin I, Cullen WK, Hu N-W, Rowan MJ: Alzheimer’s disease Aβ assemblies mediating rapid disruption of synaptic plasticity and memory. Molecular Brain. 2012, 5: 25-10.1186/1756-6606-5-25.
Kittelberger KA, Piazza F, Tesco G, Reijmers LG: Natural amyloid-beta oligomers acutely impair the formation of a contextual fear memory in mice. PLoS One. 2012, 7: e29940-10.1371/journal.pone.0029940.
Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O: Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008, 28: 14537-14545. 10.1523/JNEUROSCI.2692-08.2008.
Puzzo D, Privitera L, Fà M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Merchen M, Jung SS, Palmeri A, Arancio O: Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011, 69: 819-830. 10.1002/ana.22313.
Puzzo D, Arancio O: Amyloid-β peptide: Dr. Jekyll or Mr. Hyde?. J Alzheimers Dis. 2013, 33: S111-S120.
Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL: Synaptic targeting by Alzheimer’s-related amyloid β oligomers. J Neurosci. 2004, 24: 10191-10200. 10.1523/JNEUROSCI.3432-04.2004.
Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL: Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007, 27: 796-807. 10.1523/JNEUROSCI.3501-06.2007.
Shughrue PJ, Acton PJ, Breese RS, Zhao W-Q, Chen-Dodson E, Hepler RW, Wolfe AL, Matthews M, Heidecker GJ, Joyce JG, Villarreal SA, Kinney JJ: Anti-ADDL antibodies differentially block oligomer binding to hippocampal neurons. Neurobiol Aging. 2010, 31: 189-202. 10.1016/j.neurobiolaging.2008.04.003.
Rammes G, Hasenjager A, Sroka-Saidi K, Deussing JM, Parsons CG: Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of β-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology. 2011, 60: 982-990. 10.1016/j.neuropharm.2011.01.051.
Poling A, Paisley-Morgan K, Panos JJ, Kim E-M, O’Hare E, Cleary JP, Lesné S, Ashe KH, Porritt M, Baker L: Oligomers of the amyloid-beta protein disrupt working memory: confirmation with two behavioral procedures. Behav Brain Res. 2008, 193: 230-234. 10.1016/j.bbr.2008.06.001.
De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, Bigio EH, Jerecic J, Acton PJ, Shughrue PJ, Chen-Dodson E, Kinney GG, Klein WL: Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol Aging. 2008, 29: 1334-1347. 10.1016/j.neurobiolaging.2007.02.029.
Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ: Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA. 2011, 108: 5819-5824. 10.1073/pnas.1017033108.
Chabrier MA, Blurton-Jones M, Agazaryan AA, Nerhus JL, Martinez-Coria H, LaFerla FM: Soluble Aβ promotes wild-type tau pathology in vivo. J Neurosci. 2012, 32: 17345-17350. 10.1523/JNEUROSCI.0172-12.2012.
Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM: Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease: a link between Aβ and tau pathology. J Biol Chem. 2006, 281: 1599-1604. 10.1074/jbc.M507892200.
Small SA, Duff K: Linking Aβ and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008, 60: 534-542. 10.1016/j.neuron.2008.11.007.
Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Kandelkow E-M: Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013, 32: 2920-2937. 10.1038/emboj.2013.207.
Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE: Water-soluble Aβ (N-40, N-42) oligomers in normal and Alzheimer’s disease brains. J Biol Chem. 1996, 271: 4077-4081. 10.1074/jbc.271.8.4077.
Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL: Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003, 100: 10417-10422. 10.1073/pnas.1834302100.
Sehlin D, Englund H, Simu B, Karlsson M, Ingelsson M, Nikolajeff F, Lannfelt L, Pettersson FE: Large aggregates are the major soluble Aβ species in AD brain fractionated with density gradient ultracentrifugation. PLoS One. 2012, 7: e32014-10.1371/journal.pone.0032014.
Georganopoulou DG, Chang L, Nam J-M, Thaxton SC, Mufson EJ, Klein WL, Mirkin CA: Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc Natl Acad Sci USA. 2005, 102: 2273-2276. 10.1073/pnas.0409336102.
Gao CM, Yam AY, Wang X, Magdangal E, Salisbury C, Peretz D, Zuckermann RN, Connolly MD, Hansson O, Minthon L, Zetterberg H, Blennow K, Fedynyshyn JP, Allauzen S: Aβ40 oligomers identified as a potential biomarker for the diagnosis of Alzheimer’s disease. PLoS One. 2010, 5: e15725-10.1371/journal.pone.0015725.
Santos AN, Ewers M, Minthon L, Simm A, Silber R-E, Blennow K, Prvulovic D, Hansson O, Hampel H: Amyloid-β oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer’s disease. J Alzheimer’s Dis. 2012, 29: 171-176.
Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM, Brody DL: Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013, 73: 104-119. 10.1002/ana.23748.
Herskovits AZ, Locascio JJ, Peskind ER, Li G, Hyman BT: A luminex assay detects amyloid β oligomers in Alzheimer’s disease cerebrospinal fluid. PLoS One. 2013, 8: e67898-10.1371/journal.pone.0067898.
Glabe CG: Structural classification of toxic amyloid oligomers. J Biol Chem. 2008, 283: 29639-29643. 10.1074/jbc.R800016200.
Rahimi F, Shanmugam A, Bitan G: Structure-function relationships of pre-fibrillar protein assemblies in Alzheimer’s disease and related disorders. Curr Alzheimer Res. 2008, 5: 319-341. 10.2174/156720508784533358.
Fändrich M: Oligomeric intermediates in amyloid formation: structure determination and mechanisms of toxicity. J Mol Biol. 2012, 421: 427-440. 10.1016/j.jmb.2012.01.006.
Roher AE, Chaney MO, Kuo Y-M, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR: Morphology and toxicity of Aβ-(1-42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 1996, 271: 20631-20635. 10.1074/jbc.271.34.20631.
Hu N-W, Smith IM, Walsh DM, Rowan MJ: Soluble amyloid-β peptides potently disrupt hippocampal synaptic plasticity in the absence of cerebrovascular dysfunction in vivo. Brain. 2008, 131: 2414-2424. 10.1093/brain/awn174.
Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ: Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999, 19: 8876-8884.
Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, Francis BM, Mount HT, Mufson EJ, Salehi A, Fahnestock M: Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2009, 29: 9321-9329. 10.1523/JNEUROSCI.4736-08.2009.
Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL: Self-assembly of Aβ1-42 into globular neurotoxins. Biochem. 2003, 42: 12749-12760. 10.1021/bi030029q.
Ono K, Condron MM, Teplow DB: Structure-neurotoxicity relationships of amyloid β-protein oligomers. Proc Natl Acad Sci USA. 2009, 106: 14745-14750. 10.1073/pnas.0905127106.
Rönicke R, Klemm A, Meinhardt J, Schröder UH, Fändrich M, Reymann KG: Aβ mediated diminution of MMT reduction - an artefact of single cell culture?. PLoS One. 2008, 3: e3236-10.1371/journal.pone.0003236.
Podlisny MB, Walsh DM, Amarante P, Ostaszewski BL, Stimson ER, Maggio JE, Teplow DB, Selkoe DJ: Oligomerization of endogenous and synthetic amyloid β-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry. 1998, 37: 3602-3611. 10.1021/bi972029u.
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ: Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002, 416: 535-539. 10.1038/416535a.
Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, Agnaf OE, Hartley DM, Selkoe DJ: Certain inhibitors of synthetic amyloid β-peptide (Aβ) fibrillogenesis block oligomerization of natural Aβ and thereby rescue long-term potentiation. J Neurosci. 2005, 25: 2455-2462. 10.1523/JNEUROSCI.4391-04.2005.
Bitan G, Fradinger EA, Spring SM, Teplow DB: Neurotoxic protein oligomers - what you see is not always what you get. Amyloid. 2005, 12: 88-95. 10.1080/13506120500106958.
Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH: Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci. 2012, 32: 17401-17406. 10.1523/JNEUROSCI.3028-12.2012.
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL: Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007, 27: 2866-2875. 10.1523/JNEUROSCI.4970-06.2007.
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D: Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009, 62: 788-801. 10.1016/j.neuron.2009.05.012.
Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R: Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010, 13: 190-196. 10.1038/nn.2476.
Tamburri A, Dudilot A, Licea S, Bourgeois C, Boehm J: NMDA-receptor activation but not ion flux is required for amyloid-beta induced synaptic depression. PLoS One. 2013, 8: e65350-10.1371/journal.pone.0065350.
Malinow R: New developments on the role of NMDA receptors in Alzheimer’s disease. Curr Opin Neurobiol. 2012, 22: 559-563. 10.1016/j.conb.2011.09.001.
Takamura A, Sato Y, Watabe D, Okamoto Y, Nakata T, Kawarabayashi T, Oddo S, Laferla FM, Shoji M, Matsubara E: Sortilin is required for toxic action of Aβ oligomers (AβOs): extracellular AβOs trigger apoptosis, and intraneuronal AβOs impair degradation pathways. Life Sci. 2012, 91: 1177-1186. 10.1016/j.lfs.2012.04.038.
Zhang Y, McLaughlin R, Goodyer C, LeBlanc A: Selective cytotoxicity of intracellular amyloid β peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002, 156: 519-529. 10.1083/jcb.200110119.
Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M: Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA. 2002, 99: 13217-13221. 10.1073/pnas.172504199.
Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M: Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci USA. 2009, 106: 16877-16882. 10.1073/pnas.0908706106.
Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R: Block of long-term potentiation by naturally secreted and synthetic amyloid β-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004, 24: 3370-3378. 10.1523/JNEUROSCI.1633-03.2004.
Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX-J, Masliah E: Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLoS One. 2008, 3: e3135-10.1371/journal.pone.0003135.
Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, Ho K, Yu G-Q, Mucke L: Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011, 469: 47-52. 10.1038/nature09635.
Yu C, Nwabuisi-Heath E, Laxton K, LaDu MJ: Endocytic pathways mediating oligomeric Aβ42 neurotoxicity. Mol Neurodegener. 2010, 5: 19-10.1186/1750-1326-5-19.
Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A: Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010, 66: 739-754. 10.1016/j.neuron.2010.04.029.
Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, Gunther EC, Nygaard HB, Strittmatter SM: Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron. 2013, 79: 887-902. 10.1016/j.neuron.2013.06.036.
Paranjape GS, Gouwens LK, Osborn DC, Nishols MR: Isolated amyloid(1-42) protofibrils, but not isolated fibrils, are robust stimulators of microglia. ACS Chem Neurosci. 2012, 3: 302-311. 10.1021/cn2001238.
Ostapchenko VG, Beraldo FH, Mohammad AH, Xie Y-F, Hirata PHF, Magalhaes AC, Lamour G, Li H, Maciejewski A, Belrose JC, Teixeira BL, Fahnestock M, Ferreira ST, Cashman NR, Hajj GNM, Jackson MF, Choy W-Y, MacDonald JF, Martins VR, Prado VF, Prado MAM: The prion protein ligand, stress-inducible phosphoprotein 1, regulates amyloid-β oligomer toxicity. J Neurosci. 2013, 33: 16552-16564. 10.1523/JNEUROSCI.3214-13.2013.
Younan ND, Sarell CJ, Davies P, Brown DR, Viles JH: The cellular prion protein traps Alzheimer’s Aβ in an oligomeric form and disassembles amyloid fibers. FASEB J. 2013, 27: 1847-1858. 10.1096/fj.12-222588.
Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM: Alzheimer amyloid-β oligomer bound to post-synaptic prion protein activates Fyn to impair neurons. Nat Neurosci. 2012, 15: 1227-1235. 10.1038/nn.3178.
Larson M, Sherman MA, Amar F, Nuvolone M, Schneider JA, Bennett DA, Aguzzi A, Lesné SE: The complex PrPc-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer’s disease. J Neurosci. 2012, 32: 16857-16871. 10.1523/JNEUROSCI.1858-12.2012.
Zhao W-Q, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, Kinney GG, Shughrue PJ, Ray WJ: Inhibition of calcineurin-mediated endocytosis and α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptors prevents amyloid β oligomer-induced synaptic disruption. J Biol Chem. 2010, 285: 7619-7632. 10.1074/jbc.M109.057182.
Wu H-Y, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT: Amyloid β induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010, 30: 2636-2649. 10.1523/JNEUROSCI.4456-09.2010.
Wu H-Y, Hudry E, Hashimoto T, Uemura K, Fan Z-Y, Berezovska O, Grosskreutz CL, Bacskai BJ, Hyman BT: Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-β revealed by a novel fluorescence resonance energy transfer assay. J Neurosci. 2012, 32: 5298-5309. 10.1523/JNEUROSCI.0227-12.2012.
Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ: Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science. 2013, 341: 1399-1404. 10.1126/science.1242077.
Townsend M, Mehta T, Selkoe DJ: Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007, 282: 33305-33312. 10.1074/jbc.M610390200.
Catalano SM, Rishton G, Izzo NJ: International Patent Publication Number, WO 2013/029060 A2. Compositions and methods for treating neurodegenerative disease. 2013
Benilova I, De Strooper B: Promiscuous Alzheimer’s amyloid: yet another partner. Science. 2013, 341: 1354-1355. 10.1126/science.1244166.
Teblow DB: On the subject of rigor in the study of amyloid β-protein assembly. Alzheimers Res Ther. 2013, 5: 39-10.1186/alzrt203.
Stine WB, Jungbauer L, Yu C, LaDu MJ: Preparing synthetic Aβ in different aggregation states. Methods Mol Biol. 2011, 670: 13-32.
Ryan DA, Narrow WC, Federoff HJ, Bowers WJ: An improved method of generating consistent soluble amyloid-beta oligomer preparations for in vitro neurotoxicity studies. J Neurosci Methods. 2010, 190: 171-179. 10.1016/j.jneumeth.2010.05.001.
Zago W, Buttini M, Comery TA, Nishioka C, Gardai SJ, Seubert P, Games D, Bard F, Schenk D, Kinney GG: Neutralization of soluble, synaptotoxic amyloid β species by antibodies is epitope specific. J Neurosci. 2012, 32: 2696-2702. 10.1523/JNEUROSCI.1676-11.2012.
Yu YJ, Watts RJ: Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013, 10: 459-472. 10.1007/s13311-013-0187-4.
Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO: Structural conversion of neurotoxic amyloid-β(1-42) oligomers to fibrils. Nat Struct Mol Biol. 2010, 17: 561-567. 10.1038/nsmb.1799.
Chaney MO, Webster SD, Kuo YM, Foher AF: Molecular modeling of the Aβ1-42 peptide from Alzheimer’s disease. Protein Eng. 1998, 11: 761-767. 10.1093/protein/11.9.761.
Mastrangelo IA, Ahmed M, Sato T, Liu W, Wang C, Hough P, Smith SO: High-resolution atomic force microscopy of soluble Aβ42 oligomers. J Mol Biol. 2006, 358: 106-119. 10.1016/j.jmb.2006.01.042.
Cerf E, Sarroukh R, Tamamizu-Kato S, Breydo L, Derclaye S, Dufrêne YF, Narayanaswami V, Goormaghtigh E, Ruysschaert J-M, Raussens V: Antiparallel β-sheet: a signature structure of the oligomeric amyloid β-peptide. Biochem J. 2009, 421: 415-423. 10.1042/BJ20090379.
Hillen H, Barghorn S, Striebinger A, Labkovsky B, Müller R, Nimmrich V, Nolte MW, Perez-Cruz C, van der Auwera I, van Leuven F, van Gaalen M, Bespalov AY, Schoemaker H, Sullivan JP, Ebert U: Generation and therapeutic efficacy of highly oligomer-specific β-amyloid antibodies. J Neurosci. 2010, 30: 10369-10379. 10.1523/JNEUROSCI.5721-09.2010.
Krafft GA, Hefti F, Goure WF, Jerecic J, Iverson KS, Walicke P, Dodart J-C: ACU-193: a drug candidate antibody that selectively targets soluble Aβ oligomers. Alzheimers Dement. 2013, 9: P326-
Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Gundman M: A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurol. 2009, 73: 2061-2070. 10.1212/WNL.0b013e3181c67808.
Kerchner GA, Boxer AL: Bapineuzumab. Expert Opin Biol Ther. 2010, 10: 1121-1130. 10.1517/14712598.2010.493872.
Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M:11C-PiB PET assessment of change in fibrillar amyloid-β load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 1, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010, 9: 363-372. 10.1016/S1474-4422(10)70043-0.
Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, DeMattos RB: Safety and changes in plasma and cerebrospinal fluid amyloid β after a single administration of an amyloid β monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharm. 2010, 33: 67-73. 10.1097/WNF.0b013e3181cb577a.
Lemere CA, Masliah E: Can Alzheimer disease be prevented by amyloid-β immunotherapy?. Nat Rev Neurol. 2010, 6: 108-119. 10.1038/nrneurol.2009.219.
Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, Friedrich S, Dean RA, Gonzales C, Sethuraman G, DeMattos RB, Mohs R, Paul SM, Siemers ER: Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012, 8: 261-271. 10.1016/j.jalz.2011.09.224.
Lobello K, Ryan JM, Liu E, Rippon G, Black R: Targeting beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer’s disease. Int J Alzheimers Dis. 2012, 2012: 628070-
Prins ND, Scheltens P: Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future. Alzheimers Res Ther. 2013, 5: 56-10.1186/alzrt220.
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R: Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014, 370: 311-321. 10.1056/NEJMoa1312889.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear RH: Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014, 370: 322-333. 10.1056/NEJMoa1304839.
Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB: Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid β. J Neurosci. 2005, 25: 629-636. 10.1523/JNEUROSCI.4337-04.2005.
Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberbug I, Schenk D: Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature. 1992, 359: 325-327. 10.1038/359325a0.
DeMattos RB, Bales KR, Cummins DJ, Dodart J-C, Paul SM, Holtzman DM: Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001, 98: 8850-8855. 10.1073/pnas.151261398.
Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S, Terasaki T, Hashimoto T, Iwatsubo T: Aβ immunotherapy: intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high affinity to soluble Aβ. J Neurosci. 2009, 29: 11393-11398. 10.1523/JNEUROSCI.2021-09.2009.
Basi G, Jacobsen JS: United States Patent Application Publication Number US 2006/0198851 A1. Humanized abeta antibodies for use in improving cognition. 2006
Toolan D, Tugusheva K, Haugabook S, Feng M, McCampbell A, Hatcher N, Zhao W-Q, Gretzula C-A, Cash-Mason T, Lemaire P, Savage M, Shughrue P, Ray J, Renger J: Characterizing the selectivity of an antibody that targets a rare and unstable conformation of the Aβ peptide in Alzheimer’s disease. 2010, Unpublished internal research report, Merck & Co, [http://www.acumenpharm.com/News/Toolan.pdf]
Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T: Epitope and isotype specificities of antibodies to β-amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci USA. 2003, 100: 2023-2028. 10.1073/pnas.0436286100.
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P: Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999, 400: 173-177. 10.1038/22124.
Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, Huang J, Kohnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T: Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000, 6: 916-919. 10.1038/78682.
Basi G, Saldanha J, Yednock T: World Intellectual Property Organization International Publication Number WO 02/46237 A2. Humanized antibodies that recognize beta amyloid peptide. 2002
Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L: Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci USA. 1997, 94: 1550-1555. 10.1073/pnas.94.4.1550.
Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, Kohnson-Wood K, Kham K, Seubert P, Freedman S, Schenk D, Games D: β-Amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J Neurosci. 2005, 25: 9096-9101. 10.1523/JNEUROSCI.1697-05.2005.
Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, Gill D, Basi G, Schenk D, Seubert P, Games D: Immunotherapy reduces vascular amyloid-β in PDAPP mice. J Neurosci. 2008, 28: 6787-6793. 10.1523/JNEUROSCI.2377-07.2008.
Bard F, Fox M, Friedrich S, Seubert P, Schenk D, Kinney GG, Yednock T: Sustained levels of antibodies against Aβ in amyloid-rich regions of the CNS following intravenous dosing in human APP transgenic mice. Exp Neurology. 2012, 238: 38-43. 10.1016/j.expneurol.2012.07.022.
Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, Grundman M, Liu E: Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Nuerol. 2012, 69: 1002-1010. 10.1001/archneurol.2012.90.
Pfeifer A, Pihlgren M, Muhs A, Watts R: World Intellectual Property Organization International Publication Number WO 2008/156622 A1. Humanized antibodies to amyloid beta. 2008
Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ: An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci. 2012, 32: 9677-9689. 10.1523/JNEUROSCI.4742-11.2012.
Rosenthal A, Ponns J, Ho W-H, Grimm JM: World Intellectual Property Organization International Publication Number WO 2006/036291 A2. Antibodies directed against amyloid-beta peptide and methods using same. 2006
Carty NC, Wilcock DM, Rosenthal A, Grimm J, Pons J, Ronan V, Gottschall PE, Gordon MN, Morgan D: Intracranial administration of deglycosyoated C-terminal-specific anti-Aβ antibody efficiently clears amyloid plaques without activating microglia in amyloid-depositing transgenic mice. J Neuroinflammation. 2006, 3: 11-10.1186/1742-2094-3-11.
La Porte SL, Bollini SS, Lanz TA, Abdiche YN, Rusnak AS, Ho W-H, Kobayashi D, Harrabi O, Pappas D, Mina EW, Milici AJ, Kawbe TT, Bales K, Lin JC, Pons J: Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for treatment of Alzheimer’s disease. J Mol Biol. 2012, 421: 525-536. 10.1016/j.jmb.2011.11.047.
Nitsch R, Hock C, Esslinger C, Knobloch M, Tissot K: World Intellectual Property Organization International Publication Number WO 2008/081008 A1. Method of providing disease-specific binding molecules and targets. 2008
Dunstan R, Bussiere T, Rhodes K, Engber T, Maier M, Weinreb P, Grimm J, Nitsch R, Arustu M, Qian F, Li M: Molecular characterization and preclinical efficacy. Alzheimers Dement. 2011, 7: S457-
Englund H, Sehlin D, Johansson A-S, Nilsson LNG, Gellerfors P, Paulie S, Lannfelt L, Pettersson FE: Sensitive ELISA detection of amyloid-β protofibrils in biological samples. J Neurochem. 2007, 103: 334-345.
Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Näslund J, Lannfelt L: The ‘Artic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat Neurosci. 2001, 4: 887-893. 10.1038/nn0901-887.
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG: Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003, 300: 486-489. 10.1126/science.1079469.
Lord A, Gumucio A, Englund H, Sehlin D, Sundquist VS, Söderberg L, Möller C, Gellerfors P, Lannfelt L, Pettersson FE, Nilsson LN: An amyloid-β protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009, 36: 425-434. 10.1016/j.nbd.2009.08.007.
Gellerfors P, Lannfelt L, Sehlin D, Pettersson FE, Englund H: United States Patent Application Publication Number US 2009/0258009 A1. Protofibril selective antibodies and the use thereof. 2009
Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, Messer J, Oroszlan K, Rauchenberger R, Richter WF, Rothe C, Urban M, Bardroff M, Winter M, Nordstedt C, Loetscher H: Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis. 2012, 28: 49-69.
Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk WE, Ashford E, Yoo K, Xu Z-X, Loetscher H, Santarelli L: Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012, 69: 198-207. 10.1001/archneurol.2011.1538.
Schupf N, Tang MX, Fukuyama H, Manly J, Andrews H, Mehta P, Ravetch J, Mayeux R: Peripheral Aβ subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci USA. 2008, 105: 14052-14057. 10.1073/pnas.0805902105.
Ravetch J, Fukuyama H: World Intellectual Property Organization, International Publication Number WO 2009/065054 A3. Antibodies specific for the protofibril form of beta-amyloid protein. 2009
Pradier L, Cohen C, Blanchard V, Debeir T, Barneoud P, Canton T, Menager J, Bohme A, Rooney T, Guillet M-C, Cameron B, Shi Y, Naimi S, Ravetch J, Claudel S, Alam J: SAR228810: an antiprotofibrillar beta-amyloid antibody designed to reduce risk of amyloid-related imaging abnormalities (ARIA). Alzheimers Dement. 2013, 9: P808-P809.
Baurin N, Blanche F, Cameron B, Duchesne M, Mikol V, Naimi S, Pradier L, Shi Y: United States Patent Application Publication No. US 2012/0177639. Humanized antibodies specific to the protofibrillar form of the beta-amyloid peptide. 2012
Dodel R, Balakrishnan K, Keyvani K, Deuster O, Neff F, Andrei-Selmer L-C, Röskam S, Stüer C, Al-Abed Y, Noelker C, Balzer-Geldsetzer M, Oertel W, Du Y, Bacher M: Naturally occurring autoantibodies against β-amyloid: investigating their role in transgenic animal and in vitro models of Alzheimer’s disease. J Neurosci. 2011, 31: 5847-5854. 10.1523/JNEUROSCI.4401-10.2011.
Moir RD, Tseitlin KA, Soscia S, Hyman BT, Irizarry MC, Tanzi RE: Autoantibodies to redox-modified oligomeric Aβ are attenuated in the plasma of Alzheimer’s disease patients. J Biol Chem. 2005, 280: 17458-17463. 10.1074/jbc.M414176200.
Szabo P, Relkin N, Weksler ME: Natural human antibodies to amyloid beta peptide. Autoimmunity Rev. 2008, 7: 415-420. 10.1016/j.autrev.2008.03.007.
Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL: Monoclonal antibodies that target pathological assemblies of Aβ. J Neurochem. 2007, 100: 23-35. 10.1111/j.1471-4159.2006.04157.x.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
All authors are employees or paid consultants of Acumen Pharmaceuticals, Inc. and own stock or stock options of this company.
Authors’ contributions
All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Goure, W.F., Krafft, G.A., Jerecic, J. et al. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer’s disease immunotherapeutics. Alz Res Therapy 6, 42 (2014). https://doi.org/10.1186/alzrt272
Published:
DOI: https://doi.org/10.1186/alzrt272