Abstract
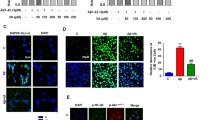
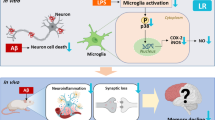
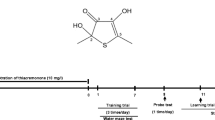
Methylene blue (MB) phase II clinical trials reported improvements in cognitive functions of Alzheimer’s disease (AD) patients. Regarding MB mechanism of action, its antioxidant and mitochondrial protective effects have been previously described. In relation to AD, it has been recently reported that MB reduced amyloid beta (Aβ) levels in AD models. The mitochondrial enzyme amyloid-binding alcohol dehydrogenase (ABAD) has been shown to bind Aβ inducing mitochondrial dysfunction, providing a direct relation between Aβ toxicity and mitochondrial dysfunction occurring in AD. Since it has been reported that inhibiting ABAD protects mitochondrial functions and prevents Aβ-induced toxicity, the aim of the current study was to investigate if the protective effects of MB could be associated with an effect on ABAD levels and functions. The current study shows that MB is able to enhance cell viability, reduce both reactive oxygen species levels and importantly Aβ oligomers in a lipopolysaccharide (LPS) mouse model. Interestingly, ABAD levels were increased in the brains of the LPS mouse model and MB treatment was able to reduce its levels. Given that regulation of the estradiol level is a well-established function of ABAD, brain estradiol level was compared in LPS mouse model and in MB-treated mice. The results of the current study show that MB treatment is able to improve significantly the LPS-induced decrease of estradiol levels in mice brains, indicating its ability to modulate both levels and function of ABAD. These results give a new insight to possible mechanisms of MB in AD.





Similar content being viewed by others
References
Herman MI, Chyka PA, Butler AY, Rieger SE (1999) Methylene blue by intraosseous infusion for methemoglobinemia. Ann Emerg Med 33(1):111–113
Wischik CM, Harrington CR, Storey JM (2014) Tau-aggregation inhibitor therapy for Alzheimer’s disease. Biochem Pharmacol 88(4):529–539. doi:10.1016/j.bcp.2013.12.008
Jinwal UK, Groshev A, Zhang J, Grover A, Sutariya VB (2014) Preparation and characterization of methylene blue nanoparticles for Alzheimer’s disease and other tauopathies. Curr Drug Deliv 11(4):541–550
O'Leary JC 3rd, Li Q, Marinec P, Blair LJ, Congdon EE, Johnson AG, Jinwal UK, Koren J 3rd, Jones JR, Kraft C, Peters M, Abisambra JF, Duff KE, Weeber EJ, Gestwicki JE, Dickey CA (2010) Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener 5:45. doi:10.1186/1750-1326-5-45
Ladiwala AR, Dordick JS, Tessier PM (2011) Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J Biol Chem 286(5):3209–3218. doi:10.1074/jbc.M110.173856
Irwin JA, Wong HE, Kwon I (2013) Different fates of Alzheimer’s disease amyloid-beta fibrils remodeled by biocompatible small molecules. Biomacromolecules 14(1):264–274. doi:10.1021/bm3016994
Medina DX, Caccamo A, Oddo S (2011) Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol 21(2):140–149. doi:10.1111/j.1750-3639.2010.00430.x
Mori T, Koyama N, Segawa T, Maeda M, Maruyama N, Kinoshita N, Hou H, Tan J, Town T (2014) Methylene blue modulates beta-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice. J Biol Chem 289(44):30303–30317. doi:10.1074/jbc.M114.568212
Paban V, Manrique C, Filali M, Maunoir-Regimbal S, Fauvelle F, Alescio-Lautier B (2014) Therapeutic and preventive effects of methylene blue on Alzheimer’s disease pathology in a transgenic mouse model. Neuropharmacology 76(Pt A):68–79. doi:10.1016/j.neuropharm.2013.06.033
Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G (2002) Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol Aging 23(3):371–376
Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U (2010) Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimer’s Dis: JAD 20(2):S499–512. doi:10.3233/JAD-2010-100504
Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH (2011) Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem 286(18):16504–16515. doi:10.1074/jbc.M110.208447
Lin AL, Poteet E, Du F, Gourav RC, Liu R, Wen Y, Bresnen A, Huang S, Fox PT, Yang SH, Duong TQ (2012) Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One 7(10):e46585. doi:10.1371/journal.pone.0046585
Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F (2004) Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav 77(1):175–181
Riha PD, Rojas JC, Gonzalez-Lima F (2011) Beneficial network effects of methylene blue in an amnestic model. NeuroImage 54(4):2623–2634. doi:10.1016/j.neuroimage.2010.11.023
Rhein V, Baysang G, Rao S, Meier F, Bonert A, Muller-Spahn F, Eckert A (2009) Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell Mol Neurobiol 29(6–7):1063–1071. doi:10.1007/s10571-009-9398-y
Readnower RD, Sauerbeck AD, Sullivan PG (2011) Mitochondria, amyloid beta, and Alzheimer’s disease. Int J Alzheimers Dis 2011:104545. doi:10.4061/2011/104545
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med 14(2):45–53. doi:10.1016/j.molmed.2007.12.002
Hernandez-Zimbron LF, Luna-Munoz J, Mena R, Vazquez-Ramirez R, Kubli-Garfias C, Cribbs DH, Manoutcharian K, Gevorkian G (2012) Amyloid-beta peptide binds to cytochrome C oxidase subunit 1. PLoS One 7(8):e42344. doi:10.1371/journal.pone.0042344
He XY, Wen GY, Merz G, Lin D, Yang YZ, Mehta P, Schulz H, Yang SY (2002) Abundant type 10 17 beta-hydroxysteroid dehydrogenase in the hippocampus of mouse Alzheimer’s disease model. Brain Res Mol Brain Res 99(1):46–53
Yang SY, He XY, Miller D (2007) HSD17B10: a gene involved in cognitive function through metabolism of isoleucine and neuroactive steroids. Mol Genet Metab 92(1–2):36–42. doi:10.1016/j.ymgme.2007.06.001
Lim YA, Grimm A, Giese M, Mensah-Nyagan AG, Villafranca JE, Ittner LM, Eckert A, Gotz J (2011) Inhibition of the mitochondrial enzyme ABAD restores the amyloid-beta-mediated deregulation of estradiol. PLoS One 6(12):e28887. doi:10.1371/journal.pone.0028887
Amtul Z, Wang L, Westaway D, Rozmahel RF (2010) Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer’s disease. Neuroscience 169(2):781–786. doi:10.1016/j.neuroscience.2010.05.031
Grimm A, Lim YA, Mensah-Nyagan AG, Gotz J, Eckert A (2012) Alzheimer’s disease, oestrogen and mitochondria: an ambiguous relationship. Mol Neurobiol 46(1):151–160. doi:10.1007/s12035-012-8281-x
He XY, Yang YZ, Schulz H, Yang SY (2000) Intrinsic alcohol dehydrogenase and hydroxysteroid dehydrogenase activities of human mitochondrial short-chain L-3-hydroxyacyl-CoA dehydrogenase. Biochem J 345(Pt 1):139–143
Muirhead KE, Borger E, Aitken L, Conway SJ, Gunn-Moore FJ (2010) The consequences of mitochondrial amyloid beta-peptide in Alzheimer’s disease. Biochem J 426(3):255–270. doi:10.1042/BJ20091941
Yao J, Du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SS (2011) Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. J Neurosci Off J Soc Neurosci 31(6):2313–2320. doi:10.1523/JNEUROSCI. 4717-10.2011
Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, Fang F, Chen JX, Yan SD, Gunn-Moore FJ (2007) Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer’s disease patients and a transgenic Alzheimer’s disease mouse model. Mol Cell Neurosci 35(2):377–382. doi:10.1016/j.mcn.2007.03.013
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H (2004) ABAD directly links abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304(5669):448–452. doi:10.1126/science.1091230
Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS (2005) ABAD enhances abeta-induced cell stress via mitochondrial dysfunction. FASEB J: Off Publ Fed Am Soc Exp Biol 19(6):597–598. doi:10.1096/fj.04-2582fje
Ren Y, Xu HW, Davey F, Taylor M, Aiton J, Coote P, Fang F, Yao J, Chen D, Chen JX, Yan SD, Gunn-Moore FJ (2008) Endophilin I expression is increased in the brains of Alzheimer disease patients. J Biol Chem 283(9):5685–5691. doi:10.1074/jbc.M707932200
de Castro IP, Martins LM, Tufi R (2010) Mitochondrial quality control and neurological disease: an emerging connection. Expert Rev Mol Med 12:e12. doi:10.1017/S1462399410001456
Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT (2008) Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 5:37. doi:10.1186/1742-2094-5-37
Abdel-Kader R, Hauptmann S, Keil U, Scherping I, Leuner K, Eckert A, Muller WE (2007) Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761). Pharmacol Res: Off J Ital Pharmacol Soc 56(6):493–502. doi:10.1016/j.phrs.2007.09.011
Stoll L, Schubert T, Muller WE (1992) Age-related deficits of central muscarinic cholinergic receptor function in the mouse: partial restoration by chronic piracetam treatment. Neurobiol Aging 13(1):39–44
Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L (2008) Mitochondrial Ca2+ overload underlies abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One 3(7):e2718. doi:10.1371/journal.pone.0002718
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci Off J Soc Neurosci 21(9):3017–3023
Pagani L, Eckert A (2011) Amyloid-beta interaction with mitochondria. Int J Alzheimers Dis 2011:925050. doi:10.4061/2011/925050
Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH (2006) Mitochondria are a direct site of Abeta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15(9):1437–1449. doi:10.1093/hmg/ddl066
Sakono M, Zako T (2010) Amyloid oligomers: formation and toxicity of abeta oligomers. FEBS J 277(6):1348–1358. doi:10.1111/j.1742-4658.2010.07568.x
Bernstein SL, Dupuis NF, Lazo ND, Wyttenbach T, Condron MM, Bitan G, Teplow DB, Shea JE, Ruotolo BT, Robinson CV, Bowers MT (2009) Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem 1(4):326–331. doi:10.1038/nchem.247
Akoury E, Pickhardt M, Gajda M, Biernat J, Mandelkow E, Zweckstetter M (2013) Mechanistic basis of phenothiazine-driven inhibition of Tau aggregation. Angew Chem Int Ed Engl 52(12):3511–3515. doi:10.1002/anie.201208290
Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, Gestwicki JE, Dickey CA, Yu WH, Duff KE (2012) Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 8(4):609–622. doi:10.4161/auto.19048
Spires-Jones TL, Friedman T, Pitstick R, Polydoro M, Roe A, Carlson GA, Hyman BT (2014) Methylene blue does not reverse existing neurofibrillary tangle pathology in the rTg4510 mouse model of tauopathy. Neurosci Lett 562:63–68. doi:10.1016/j.neulet.2014.01.013
Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN (2008) Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J: Off Publ Fed Am Soc Exp Biol 22(3):703–712. doi:10.1096/fj.07-9610com
Eckert GP, Renner K, Eckert SH, Eckmann J, Hagl S, Abdel-Kader RM, Kurz C, Leuner K, Muller WE (2012) Mitochondrial dysfunction—a pharmacological target in Alzheimer’s disease. Mol Neurobiol 46(1):136–150. doi:10.1007/s12035-012-8271-z
Eckert A, Schmitt K, Gotz J (2011) Mitochondrial dysfunction—the beginning of the end in Alzheimer’s disease? Separate and synergistic modes of tau and amyloid-beta toxicity. Alzheimers Res Ther 3(2):15. doi:10.1186/alzrt74
Atamna H (2009) Amino acids variations in amyloid-beta peptides, mitochondrial dysfunction, and new therapies for Alzheimer’s disease. J Bioenerg Biomembr 41(5):457–464. doi:10.1007/s10863-009-9246-2
Atamna H, Kumar R (2010) Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimer’s Dis: JAD 20(2):S439–452. doi:10.3233/JAD-2010-100414
Poteet E, Winters A, Yan LJ, Shufelt K, Green KN, Simpkins JW, Wen Y, Yang SH (2012) Neuroprotective actions of methylene blue and its derivatives. PLoS One 7(10):e48279. doi:10.1371/journal.pone.0048279
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from abeta 1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95(11):6448–6453
Reddy PH (2006) Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol 2006(3):31372. doi:10.1155/JBB/2006/31372
Yan SD, Fu J, Soto C, Chen X, Zhu H, Al-Mohanna F, Collison K, Zhu A, Stern E, Saido T, Tohyama M, Ogawa S, Roher A, Stern D (1997) An intracellular protein that binds amyloid-beta peptide and mediates neurotoxicity in Alzheimer’s disease. Nature 389(6652):689–695. doi:10.1038/39522
Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD (2006) Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci 7:74. doi:10.1186/1471-2202-7-74
Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J (2008) Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 149(6):3167–3175. doi:10.1210/en.2007-1227
Callaway NL, Riha PD, Wrubel KM, McCollum D, Gonzalez-Lima F (2002) Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosci Lett 332(2):83–86
Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F (2005) Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur J Pharmacol 511(2–3):151–158. doi:10.1016/j.ejphar.2005.02.001
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zakaria, A., Hamdi, N. & Abdel-Kader, R.M. Methylene Blue Improves Brain Mitochondrial ABAD Functions and Decreases Aβ in a Neuroinflammatory Alzheimer’s Disease Mouse Model. Mol Neurobiol 53, 1220–1228 (2016). https://doi.org/10.1007/s12035-014-9088-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9088-8