Abstract
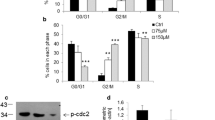
T-2 toxin is the most toxic trichothecene and a frequent contaminant in many agriculture products. Dietary ingestion represents the most common route of T-2 toxin exposure in humans. T-2 toxin exposure leads to many pathological conditions like nervous disorders, cardiovascular alterations, immune depression and dermal inflammation. However, the neuronal toxicity of T-2 toxin in vitro remains unclear. In the present study, we investigated the mechanism of T-2 toxin-induced apoptosis in human neuroblastoma cells (IMR-32). T-2 toxin was cytotoxic at a low concentration of 10 ng/ml. The 50 % inhibitory concentration (IC50) of T-2 toxin was found to be 40 ng/ml as assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, crystal violet dye exclusion test and lactate dehydrogenase (LDH) leakage. T-2 toxin increased intracellular reactive oxygen species generation as early as 15 min and peaked at 60 min as analyzed by flow cytometry. Annexin V + propidium iodide staining showed time-dependent increase in percent apoptotic cells. DNA gel electrophoresis showed oligonucleosomal DNA fragmentation typical of apoptotic cells. Additionally, casapse-3 activation and PARP cleavage indicated involvement of mitochondrial mediated caspase-dependent pathway of apoptosis. Cell cycle analysis revealed time-dependent increase in sub-G1 population of cells and significant up-regulation of CDK2, CDK6, cyclin A and p21 messenger RNA (mRNA) levels. Exposure to T-2 toxin induced the phosphorylation of extracellular signal-regulated kinase (ERK), p38-mitogen-activated protein kinase and c-jun N-terminal kinases (JNK). Analysis of human phospho-mitogen-activated protein kinase (MAPK) antibody array revealed time-dependent increase in phosphorylation. Upstream of ERK pathway Grb2, Ras and Raf and downstream transcription factors c-fos and c-jun were significantly up-regulated. Z-VAD-FMK and MAPK inhibitors (PD 98059, SB 203580 and ZM 336372) exposure prior to T-2 toxin treatment significantly decreased percent of apoptotic cells compared to only T-2 toxin-exposed cells. Results of the present study show that T-2 toxin at nanogram concentrations can induce apoptosis in human neuronal cells through multiple signal transduction pathways. The study provides possible leads for developing therapeutic approaches to prevent T-2 toxin-induced neurotoxicity.












Similar content being viewed by others
Abbreviations
- CVDE:
-
Crystal violet dye exclusion
- CHAPS:
-
3[(3-Cholamidopropyl) dimethlylammonio]-1-propanesulfate
- PARP:
-
Poly(ADP-ribose) polymerase
- PMSF:
-
Phenylmethylsulfonyl fluoride
- DTT:
-
Dithiothreitol
- PBS:
-
Phosphate-buffered saline
- DCF-DA:
-
2,7-dichlorofluorescein diacetate
- LDH:
-
Lactate dehydrogenase
- MAPK:
-
Mitogen-activated protein kinase
- ROS:
-
Reactive oxygen species
- PI:
-
Propidium iodide
References
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16:497–516
Li M, Harkema JR, Islam Z, Cuff CF, Pestka J (2006) T-2 toxin impairs murine immune response to respiratory reovirus ans exacerbates viral broncholitis. Toxicol Appl Pharmacol 217:76–85
Bunner DL, Morris ER (1988) Alteration of multiple cell membrane functions in L-6 myoblasts by T-2 toxin: an important mechanism of action. Toxicol Appl Pharmacol 92:113–121
Doi K, Ishigama N, Sehata S (2008) T-2 toxin induced toxicity in pregnanat mice and rats. Int J Mol Sci 9:2146-2158.
Schothorst RC, van Egmond (2004) Report from SCOOP task 3.2.10 “Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population ofEU member states” Toxicol Lett 153:133-143.
EFSA (2011) Scientific opinion on the risks for animal and public helath related presence of T-2 and HT-2 toxin in food and feed. EFSA Journal 12
Sehata S, Kiyosawa N, Atsumi F et al (2005) Microarray analysis of T-2 toxin-induced liver, placenta and fetal liver lesions in pregnant rats. Exp Toxicol Pathol 57:15–28
Watson SA, Mirocha CJ, Hayes AW (1984) Analysis for trichothecenes in samples from Southeast Asia associated with “Yellow Rainˮ. Fundam Appl Toxicol 4:700–717
Chaudhary M, Lakshmana Rao PV (2010) Brain oxidative stress after dermal and subcutaneous exposure of T-2 toxin in mice. Food Chem Toxicol 48:3436–3442
Jayaraj R, Agrawal M, Gupta N, Lakshmana Rao PV (2011) Alteration of blood brain barrier permeability by T-2 toxin: role of MMP-9 and inflammatory cytokines. Toxicology 280:44–52
Weidner M, Lenczyk M, Schwerdt G, Gekle M, Humpf H-U (2013) Neurotoxic potential and cellular uptake of T-2 toxin in human astrocytes in primary culture. Chemical Res Toxicol 26:347–355
Weidner M, Huwel S, Ebert F, Schwerdtle T, Galla H-J (2013) Influence of T-2 and HT-2 toxin on the blood brain barrier in vitro: new experimental hints for neurotoxic effects. Plos One 8(3):e60484. doi:10.1371/journal.pone.0060484
Chaudhari M, Jayaraj R, Bhaskar AS, Lakshmana Rao PV (2009) Oxidative stress induction by T-2 toxin causes DNA damage and triggers apoptosis via caspase pathway in human cervical cancer cells. Toxicology 262:153–161
LePage KT, Dickey RW, Gerwick WH, Jester EL, Murray TF (2005) On the use of neuro-2a neuroblastoma cells versus intact neuron in primary culture for neurotoxicity studies. Critical Rev Neurobiol 17:27–50
Erlejman AG, Otezia PI (2002) The oxidant defence system in human neuroblastoma IMR-32 cells pre differentiation and post-differentiation to neuronal phenotypes. Neurochem Res 27:1499–506
Gong J, Traganos F, Darzynkiewicz Z (1994) A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem 218:314–319
Tellman G, Olivier G (2006) Light cycler 480 real Time PCR System: innovative solutions for relative quantification. Biogeosciences 4:16–18
Li Y, Wang Z, Beier RC, Shen J, Smet DD, De Saeger S, Zhang S (2011) T-2 toxin, a trichothecene mycotoxin: review of toxicity, metabolism, and analytical methods. J Agri Food Chem 59:3441–3453
Gutleb AC, Morrison E, Murk AJ (2002) Cytotoxicity assays for mycotoxins produced by Fusarium strains: a review. Environ Toxicol Pharmacol 11:309–320
Doi K, Ishigami N, Sehata S (2008) T-2 toxin induced toxicity in pregnant mice and rats. Int J Mol Sci 9:2146-2158.
Bouaziz C, Sharaf El Dein O, El Golli E, Abid-Essefi S, Brenner C, Lemaire C, Bacha H (2008) Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 254:19–28
Daugas E, Susin SA, Zamzami N, Ferri KF, Irinipoulou T, Larochite N, Prevost M-C, Lebr B, Andrews D, Penninger J, Kroemer G (2000) Mitochondria-nuclear translocation of AIF in apoptosis and necrosis. FASEB J 14:729–739
Liu B, Chen Y, St Clair DK (2008) ROS and p53: a versatile partnership. Free Radical Biol Med 44:1529–1535
Jayaraj R, Gupta N, Agrawal M, Bhaskar ASB, Lakshmana Rao PV (2011) Modulation of ROS/MAPK signaling pathway by okadaic acid leads to cell death via, mitochondrial mediated caspase-dependent mechanism. Apoptosis 16:145–161
Deguin-Chambon V, Vacher M, Jullien M, May E, Bourdon J-C (2000) Direct transactivation of c-Ha-ras gene by p53: evidence for its involvement in p53 transactivation activity and p53-mediated apoptosis. Oncogene 19:5831–5841
Satyanarayana A, Hilton MB, Kadis P (2008) p21 inhibits CDK1 in the absence of CDK2 to maintain G1/S phase DNA damage checkpoint. Mol Biol Cell 19:65–77
Meikrantz W, Schlegel R (1995) Apoptosis and the cell cycle. J Cell Biochem 58:160–174
Mundy WR, Freudenrich TM (2006) Apoptosis of cerebellar granule cells induced by organotin compounds found in drinking water: involvement of MAP kinases. Neurotoxicology 27:71–81
Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802:396–405
Tsai CW, Lin CY, Lin HH, Chen JH (2011) Carnosic acid, a rosemary phenolic compound induces apoptosis through reactive oxygen species-mediated p38 activation in human neuroblastoma IMR-32 cells. Neurochem Res 36:2442–2451
Zago MP, Mackenzie GG, Adamo AM, Keen CL, Oteiza PI (2005) Differential modulation of Map kinases by zinc deficiency in IMR-32 cells : role of H2O2. Antioxid Redox Signal 7:1773–1782
Lo YYC, Cruz TF (1995) Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem 270:11727–11730
Paula Monje, Hernandez-Losa, Lyons RJ, Castellone, Gutkind JS (2005) Regulation of the transcriptional activity of c-fos by ERK a novel role for the prolyl isomerase Pin1 280: 35801-35804
Shinozuka J, Suzuki H, Tsutsui S, Nakayama H, Doi K (2001) T-2 toxin-induced apoptosis and c-fos mRNA expression in ConA-stimulated mouse thymocyte primary culture. J Toxicol Pathol 14:247–251
Marchetti A, Cecchinelli B, Angelo MD, Orazi GD, Crescenzi M, Sacchi A, Soddu S (2004) p53 can inhibit cell proliferation through caspase-mediated cleavage of ERK2/MAPK. Cell Death Diff 11:596–607
Acknowledgments
The authors thank Dr. M. P. Kaushik, Director, DRDE, for providing the necessary facilities and encouragement, and Ms. Mona Agrawal is thankful to CSIR for the award of Senior Research Fellowship.
Conflict of Interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrawal, M., Bhaskar, A.S.B. & Rao, P.V.L. Involvement of Mitogen-Activated Protein Kinase Pathway in T-2 Toxin-Induced Cell Cycle Alteration and Apoptosis in Human Neuroblastoma Cells. Mol Neurobiol 51, 1379–1394 (2015). https://doi.org/10.1007/s12035-014-8816-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8816-4