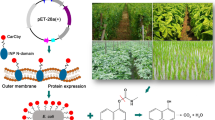
Abstract
Laccase CotA from Bacillus subtilis 168 was successfully displayed on the membrane of Escherichia coli cells using poly-γ-glutamate synthetase A protein (PgsA) from B. subtilis as an anchoring matrix. Further analyses demonstrated that the fusion protein PgsA/CotA efficiently translocates to the cell surface of E. coli with an enzymatic activity of 65 U/108 cells. Surface-displayed CotA was shown to possess improved enzymatic properties compared with those of the wild-type CotA, including higher thermal stability (above 90% activity at 70 °C and nearly 40% activity at 90 °C after 5-h incubation) and stronger inhibitor tolerance (approximately 80 and 65% activity when incubated with 200 and 400 mM NaCl, respectively). Furthermore, the whole-cell system was demonstrated to have high enzymatic activity against anthraquinone dye, Acid Blue 62, triphenylmethane dye, Malachite Green, and azo dye, Methyl Orange with the decolorization percentages of 91, 45, and 75%, after 5-h incubation, respectively.





Similar content being viewed by others
References
Wang, W., Zhang, Z., Ni, H., Yang, X. M., Li, Q. Q., & Li, L. (2012) Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase. Microbial Cell Factories, 11, 75
Forgacs, E., Cserhati, T., & Oros, G. (2004). Removal of synthetic dyes from wastewaters: A review. Environment International, 30, 953–971.
Mishra, G., & Tripathy, M. (1993). A critical review of the treatments for decolourization of textile effluent. Colourage, 40, 35–35.
Dos Santos, A. B., Cervantes, F. J., & van Lier, J. B. (2007). Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresource Technology, 98, 2369–2385.
Fang, Z. M., Li, T. L., Chang, F., Zhou, P., Fang, W., Hong, Y. Z., Zhang, X. C., Peng, H., & Xiao, Y. Z. (2012). A new marine bacterial laccase with chloride-enhancing, alkaline-dependent activity and dye decolorization ability. Bioresource Technology, 111, 36–41.
Santos, A., Mendes, S., Brissos, V., & Martins, L. O. (2014). New dye-decolorizing peroxidases from Bacillus subtilis and Pseudomonas putida MET94: Towards biotechnological applications. Applied Microbiology and Biotechnology, 98, 2053–2065.
Pan, K., Zhao, N., Yin, Q., Zhang, T., Xu, X., Fang, W., Hong, Y., Fang, Z., & Xiao, Y. (2014). Induction of a laccase Lcc9 from Coprinopsis cinerea by fungal coculture and its application on indigo dye decolorization. Bioresource Technology, 162, 45–52.
Si, J., Peng, F., & Cui, B. (2013). Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens. Bioresource Technology, 128, 49–57.
Baldrian, P. (2006). Fungal laccases—occurrence and properties. FEMS Microbiology Reviews, 30, 215–242.
Giardina, P., Faraco, V., Pezzella, C., Piscitelli, A., Vanhulle, S., & Sannia, G. (2010). Laccases: A never-ending story. Cellular and Molecular Life Sciences: CMLS, 67, 369–385.
Singh, G., Bhalla, A., Kaur, P., Capalash, N., & Sharma, P. (2011). Laccase from prokaryotes: A new source for an old enzyme. Reviews in Environmental Science and Bio/Technology, 10, 309–326.
Chandra, R., & Chowdhary, P. (2015). Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environmental Science. Processes & Impacts, 17, 326–342.
Beneyton, T., Coldren, F., Baret, J. C., Griffiths, A. D., & Taly, V. (2014). CotA laccase: High-throughput manipulation and analysis of recombinant enzyme libraries expressed in E. coli using droplet-based microfluidics. The Analyst, 139, 3314–3323.
Hullo, M. F., Moszer, I., Danchin, A., & Martin-Verstraete, I. (2001). CotA of Bacillus subtilis is a copper-dependent laccase. Journal of Bacteriology, 183, 5426–5430.
Dawkar, V. V., Jadhav, U. U., Jadhav, S. U., & Govindwar, S. P. (2008). Biodegradation of disperse textile dye Brown 3REL by newly isolated Bacillus sp. VUS. Journal of Applied Microbiology, 105, 14–24.
Brissos, V., Pereira, L., Munteanu, F. D., Cavaco-Paulo, A., & Martins, L. O. (2009). Expression system of CotA-laccase for directed evolution and high-throughput screenings for the oxidation of high-redox potential dyes. Biotechnology Journal, 4, 558–563.
Pereira, L., Coelho, A. V., Viegas, C. A., Santos, M. M., Robalo, M. P., & Martins, L. O. (2009). Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. Journal of Biotechnology, 139, 68–77.
Guan, Z. B., Shui, Y., Song, C. M., Zhang, N., Cai, Y. J., & Liao, X. R. (2015). Efficient secretory production of CotA-laccase and its application in the decolorization and detoxification of industrial textile wastewater. Environmental Science and Pollution Research International, 22, 9515–9523.
Van Bloois, E., Winter, R. T., Kolmar, H., & Fraaije, M. W. (2011). Decorating microbes: Surface display of proteins on Escherichia coli. Trends in Biotechnology, 29, 79–86.
Li, W., Shi, H., Ding, H., Wang, L., Zhang, Y., Li, X., & Wang, F. (2015). Cell surface display and characterization of Rhizopus oryzae lipase in Pichia pastoris using Sed1p as an anchor protein. Current Microbiology, 71(1), 150–155.
Yang, C., Cai, N., Dong, M., Jiang, H., Li, J., Qiao, C., & Chen, W. (2008). Surface display of MPH on Pseudomonas putida JS444 using ice nucleation protein and its application in detoxification of organophosphates. Biotechnology and Bioengineering, 99(1), 30–37.
Bertrand, B., Trejo-Hernández, M. R., Morales-Guzmán, D., Caspeta, L., Suárez, R. R., & Martínez-Morales, F. (2016). Functional expression, production, and biochemical characterization of a laccase using yeast surface display technology. Fungal Biology, 120(12), 1609.
Bleve, G., Lezzi, C., Spagnolo, S., Rampino, P., Perrotta, C., & Mita, G., et al. (2014). Construction of a laccase chimerical gene: Recombinant protein characterization and gene expression via yeast surface display. Applied Biochemistry & Biotechnology, 172(6), 2916–2931.
Nakanishi, A., Bae, J. G., Fukai, K., Tokumoto, N., Kuroda, K., & Ogawa, J., et al. (2012). Effect of pretreatment of hydrothermally processed rice straw with laccase-displaying yeast on ethanol fermentation. Applied Microbiology & Biotechnology, 94(4), 939–948.
Schuurmann, J., Quehl, P., Festel, G., & Jose, J. (2014). Bacterial whole-cell biocatalysts by surface display of enzymes: Toward industrial application. Applied Microbiology and Biotechnology, 98, 8031–8046.
Lee, S. Y., Choi, J. H., & Xu, Z. (2003) Microbial cell surface display. Trends in Biotechnology, 21, 45–52
Narita, J., Okano, K., Tateno, T., Tanino, T., Sewaki, T., Sung, M. H., Fukuda, H., & Kondo, A. (2006). Display of active enzymes on the cell surface of Escherichia coli using PgsA anchor protein and their application to bioconversion. Applied Microbiology and Biotechnology, 70, 564–572.
Liu, Y., Zhang, R., Lian, Z., Wang, S., & Wright, A. T. (2014). Yeast cell surface display for lipase whole cell catalyst and its applications. Journal of Molecular Catalysis B-Enzymatic, 106, 17–25.
Gupta, N., & Farinas, E. T. (2010). Directed evolution of CotA laccase for increased substrate specificity using Bacillus subtilis spores. Protein Engineering, Design & Selection: PEDS, 23, 679–682.
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2011). Bacterial decolorization and degradation of azo dyes: A review. Journal of the Taiwan Institute of Chemical Engineers, 42, 138–157.
Chen, H., Zhang, T., Jia, J., Vastermark, A., Tian, R., Ni, Z., Chen, Z., Chen, K., & Yang, S. (2015). Expression and display of a novel thermostable esterase from Clostridium thermocellum on the surface of Bacillus subtilis using the CotB anchor protein. Journal of Industrial Microbiology & Biotechnology, 42, 1439–1448.
Brander, S., Mikkelsen, J. D., & Kepp, K. P. (2014). Characterization of an alkali- and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS ONE, 9, e99402.
Chakroun, H., Mechichi, T., Martinez, M. J., Dhouib, A., & Sayadi, S. (2010). Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: Application on bioremediation of phenolic compounds. Process Biochemistry, 45, 507–513.
Huang, W.-T., Tai, R., Hseu, R.-S., & Huang, C.-T. (2011). Overexpression and characterization of a thermostable, pH-stable and organic solvent-tolerant Ganoderma fornicatum laccase in Pichia pastoris. Process Biochemistry, 46, 1469–1474.
Ruijssenaars, H. J., & Hartmans, S. (2004). A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Applied Microbiology and Biotechnology, 65, 177–182.
Brissos, V., Chen, Z., & Martins, L. O. (2012). The kinetic role of carboxylate residues in the proximity of the trinuclear centre in the O2 reactivity of CotA-laccase. Dalton Transactions, 41, 6247–6255.
Enguita, F. J., Martins, L. O., Henriques, A. O., & Carrondo, M. A. (2003). Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. The Journal of Biological Chemistry, 278, 19416–19425.
Ueda, M. (2016). Establishment of cell surface engineering and its development. Bioscience Biotechnology And Biochemistry, 80, 1243–1253.
Li, W., Shi, H., Ding, H., Wang, L., Zhang, Y., Li, X., & Wang, F. (2015). Cell surface display and characterization of Rhizopus oryzae Lipase in Pichia pastoris using Sed1p as an anchor protein. Current Microbiology, 71, 150–155.
Luke, K. A., Higgins, C. L., & Wittung-Stafshede, P. (2007). Thermodynamic stability and folding of proteins from hyperthermophilic organisms. The FEBS Journal, 274, 4023–4033.
Hsueh, C.-C., Chen, B.-Y., & Yen, C.-Y. (2009). Understanding effects of chemical structure on azo dye decolorization characteristics by Aeromonas hydrophila. Journal of Hazardous Materials, 167, 995–1001.
Pearce, C. (2003). The removal of colour from textile wastewater using whole bacterial cells: A review. Dyes and Pigments, 58, 179–196.
Soares, G. M., de Amorim, M. P., & Costa-Ferreira, M. (2001). Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue R. Journal of Biotechnology, 89, 123–129.
Hou, H., Zhou, J., Wang, J., Du, C., & Yan, B. (2004). Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochemistry, 39, 1415–1419.
Lu, L., Zhao, M., Zhang, B. B., Yu, S. Y., Bian, X. J., Wang, W., & Wang, Y. (2007). Purification and characterization of laccase from Pycnoporus sanguineus and decolorization of an anthraquinone dye by the enzyme. Applied Microbiology and Biotechnology, 74, 1232–1239.
Santhanam, N., Vivanco, J. M., Decker, S. R., & Reardon, K. F. (2011). Expression of industrially relevant laccases: Prokaryotic style. Trends in Biotechnology, 29, 480–489.
Zhuo, R., Ma, L., Fan, F., Gong, Y., Wan, X., Jiang, M., Zhang, X., & Yang, Y. (2011). Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp.En3 and cloning and functional analysis of its laccase gene. Journal of Hazardous Materials, 192, 855–873.
Dos Santos, L., Climent, V., Blanford, C. F., & Armstrong, F. A. (2010). Mechanistic studies of the ‘blue’ Cu enzyme, bilirubin oxidase, as a highly efficient electrocatalyst for the oxygen reduction reaction. Physical Chemistry Chemical Physics: PCCP, 12, 13962–13974.
Johannes, C., & Majcherczyk, A. (2000). Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Applied and Environmental Microbiology, 66, 524–528.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31700092, No. 21727818, No. 21390200, No. 21706125, No. 21706124), the Jiangsu Province Natural Science Foundation for Youths (No. BK20170997, No. BK20170993), the Project of State Key Laboratory of Materials Oriented Chemical Engineering (KL17-09) and the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (XTE1834), the open foundation of Jiangsu Key Laboratory for Biomass-Based Energy and Enzyme Technology, (BEETKB1801).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Dong, W., Lv, Z. et al. Surface Display of Bacterial Laccase CotA on Escherichia coli Cells and its Application in Industrial Dye Decolorization. Mol Biotechnol 60, 681–689 (2018). https://doi.org/10.1007/s12033-018-0103-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-018-0103-6