Abstract
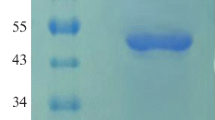
The codon-optimized phytase gene of the thermophilic mold Sporotrichum thermophile (St-Phy) was expressed in Pichia pastoris. The recombinant P. pastoris harboring the phytase gene (rSt-Phy) yielded a high titer of extracellular phytase (480 ± 23 U/mL) on induction with methanol. The recombinant phytase production was ~40-fold higher than that of the native fungal strain. The purified recombinant phytase (rSt-Phy) has the molecular mass of 70 kDa on SDS-PAGE, with K m and V max (calcium phytate), k cat and k cat/K m values of 0.147 mM and 183 nmol/mg s, 1.3 × 103/s and 8.84 × 106/M s, respectively. Mg2+ and Ba2+ display a slight stimulatory effect, while other cations tested exert inhibitory action on phytase. The enzyme is inhibited by chaotropic agents (guanidinium hydrochloride, potassium iodide, and urea), Woodward’s reagent K and 2,3-bunatedione, but resistant to both pepsin and trypsin. The rSt-Phy is useful in the dephytinization of broiler feeds efficiently in simulated gut conditions of chick leading to the liberation of soluble inorganic phosphate with concomitant mitigation in antinutrient effects of phytates. The addition of vanadate makes it a potential candidate for generating haloperoxidase, which has several applications.




Similar content being viewed by others
References
Akita, M., Kayatama, K., Hatada, Y., Ito, S., & Horikoshi, K. (2005). A novel β-glucanase gene from Bacillus halodurans C-125. FEMS Microbiology Letters, 248, 9–15.
Arjmand, S., Lotfi, A. S., Shamsara, M., & Mowla, S. J. (2013). Elevating the expression level of biologically active recombinant human alpha 1-antitrypsin in Pichia pastoris. Electronic Journal of Biotechnology,. doi:10.2225/vol16-issue1-fulltext-4.
Bohn, L., Meyer, A. S., & Rasmussen, S. K. (2008). Phytate: Impact on environment and human nutrition a challenge for molecular breeding. Journal of Zheijang University Science B, 9, 165–191.
Casey, A., & Walsh, G. (2004). Identification and characterization of a phytase of potential commercial interest. Journal of Biotechnology, 110, 313–322.
Cereghino, G., Sunga, A., Cereghino, J. L., & Cregg, J. M. (2002). Genetic engineering. US: Springer.
Chauthaiwale, J., & Rao, M. (1994). Chemical modification of xylanase from alkalothermophilic Bacillus species: Evidence for essential carboxyl group. Biochimica et Biophysica Acta, 1204, 164–168.
Cregg, J. M., Cereghino, J. L., Shi, J. Y., & Higgins, D. R. (2000). Recombinant protein expression in Pichia pastoris. Molecular Biotechnology, 16, 23–52.
Delroisse, J. M., et al. (2005). Expression of a synthetic gene encoding a Tribolium castaneum carboxylesterase in Pichia pastoris. Protein Expression and Purification, 42, 286–294.
Dixon, M., & Webb, E. C. (1979). Enzyme kinetics. In M. Dixon & E. C. Webb (Eds.), Enzymes (pp. 47–206). New York: Academic Press.
Ehrlich, K. C., Montalbano, B. G., Mullaney, E. J., Dischinger, H. C., & Ullah, A. H. J. (1993). Identification and cloning of a second phytase gene (phyB) from Aspergillus niger. Biochemical and Biophysical Research Communications, 195, 53–57.
Fonseca-Maldonado, R., Maller, A., Bonneil, E., Thibault, P., Botelho-Machado, C., Ward, R. J., & Polizeli Mde, L. (2014). Biochemical properties of glycosylation and characterization of a histidine acid phosphatase (phytase) expressed in Pichia pastoris. Protein Expression and Purification, 99, 43–49.
Fu, D., Li, Z. Y., Huang, H. Q., Yuan, T., Shi, P., Luo, H., et al. (2011). Catalytic efficiency of HAP phytases is determined by a key residue in close proximity to the active site. Applied Microbiology and Biotechnology, 90, 1295–1302.
Gulati, H. K., Chadha, B. S., & Saini, H. S. (2007). Production, purification and characterization of thermostable phytase from thermophilic fungus Thermomyces lanuginosus TL-7. Acta Microbiologica et Immunologica Hungarica, 54, 121–138.
Gustafsson, C., Govindarajan, S., & Minshull, J. (2004). Codon bias and heterologous protein expression. Trends in Biotechnology, 22, 346–353.
Hemrika, W., Renirie, R., Dekker, H. L., Barnett, P., & Wever, R. (1997). From phosphatases to vanadium peroxidases: A similar architecture of the active site. PNAS USA, 94, 2145–2149.
Hunter-Cevera, J. C., & Sotos, L. (1986). Screening for a “new” enzyme in nature: Haloperoxidase production by Death Valley dematiaceous hyphomycetes. Microbial Ecology, 12, 121–127.
Joshi, S., & Satyanarayana, T. (2014). Optimization of heterologous expression of the phytase (PPHY) of Pichia anomala in P. pastoris and its applicability in fractionating allergenic glycinin from soy protein. Journal of Industrial Microbiology and Biotechnology, 41, 977–987.
Joshi, S., & Satyanarayana, T. (2015). Characteristics and applicability of phytase of the yeast Pichia anomala in synthesizing haloperoxidase. Applied Biochemistry and Biotechnology,. doi:10.1007/s12010-015-1650-y.
Kanekiyo, M., et al. (2005). Mycobacterial codon optimization enhances antigen expression and virus-specific immune responses in recombinant mycobacterium bovis bacille calmette- gue´rin expressing human immunodeficiency virus type 1gag. Journal of Virology, 79, 8716–8723.
Kanovsky, J. R. (1984). Singlet oxygen production by chloroperoxidase-hydrogen peroxide-halide systems. Journal of Biological Chemistry, 259, 5596–5600.
Kaur, P., Singh, B., Böer, E., Straube, N., Piontek, M., Satyanarayana, T., & Kunze, G. (2010). Pphy—A cell-bound phytase from the yeast Pichia anomala: Molecular cloning of the gene PPHY and characterization of the recombinant enzyme. Journal of Biotechnology, 149, 8–15.
Kerovuo, J., Lauraeus, M., Nurminen, P., Kalkkinen, N., & Apajalahti, J. (1998). Isolation, characterization, molecular gene cloning and sequencing of a novel phytase from Bacillus subtilis. Applied and Environmental Microbiology, 64, 2079–2085.
Komissarov, A. A., Romanova, D. V., & Debabov, V. G. (1995). Complete inactivation of Escherichia coli uridine phosphorylase by modification of asp with Woodwards reagent k. Journal of Biological Chemistry, 270, 10050–10055.
Konietzny, U., & Greiner, R. (2002). Molecular and catalytic properties of phytate degrading enzymes (phytases). International Journal of Food Science & Technology, 37, 791–812.
Kumar, V., & Satyanarayana, T. (2013). Biochemical and thermodynamic characteristics of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles, 17, 797–808.
Lammertyn, E., Van, M. L., Bijnens, A. P., Joris, B., & Anné, J. (1996). Codon adjustment to maximise heterologous gene expression in Streptomyces lividans can lead to decreased mRNA stability and protein yield. Molecular and General Genetics, 250, 223–229.
Lee, C., Lee, S., Shin, S. G., & Hwang, S. (2008). Real-time PCR determination of rRNA gene copy number: Absolute and relative quantification assays with Escherichia coli. Applied Microbiology and Biotechnology, 78, 371–376.
Lee, S., Kim, T., Stahl, C. H., & Lei, X. G. (2005). Expression of Escherichia coli AppA2 phytase in four yeast systems. Biotechnology Letter, 27, 327–334.
Liu, S. Y., et al. (2015). Effects of 500 and 1000 FTU/kg phytase supplementation of maize based diets with two tiers of nutrient specifications on performance of broiler chickens. Animal Feed Science and Technology,. doi:10.1016/j.anifeedsci.2015.06.002.
Mansur, M., et al. (2005). Multiple gene copy number enhances insulin precursor secretion in the yeast Pichia pastoris. Biotechnology Letter, 27, 339–345.
Nampoothiri, K. M., Tomes, G. J., Roopesh, K., Szakacs, G., Nagy, V., Soccol, C. R., & Pandey, A. (2004). Thermostable phytase production by Thermoascus aurantiacus in submerged fermentation. Applied Biochemistry and Biotechnology, 118, 205–214.
Oka, C., et al. (1999). Human lysozyme secretion increased by alpha-factor pro-sequence in Pichia pastoris. Bioscience, Biotechnology and Biochemistry, 63, 1977–1983.
Outchkourov, N. S., Stiekema, W. J., & Jongsma, M. A. (2002). Optimization of the expression of equistatin in Pichia pastoris. Protein Expression and Purification, 24, 18–24.
Paoli, P., Fiaschi, T., Cirri, P., Camici, G., Manao, G., Cappugi, G., et al. (1997). Mechanism of acylphosphatase inactivation by Woodward’s reagent k. Journal of Biochemistry, 328, 855–861.
Pasamontes, L., Haiker, M., Wyss, M., Tessier, M., & Van Loon, A. (1997). Gene cloning, purification, and characterization of a heat-stable phytase from the fungus Aspergillus fumigatus. Applied and Environmental Microbiology, 63, 1696–1700.
Raboy, V. (2003). Myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry, 64, 1033–1043.
Rodriguez, E., Mullaney, E. J., & Lei, X. G. (2000). Expression of the Aspergillus fumigatus Phytase Gene in Pichia pastoris and Characterization of the Recombinant Enzyme. Biochemical and Biophysical Research Communications, 268, 373–378.
Sariyska, M. V., Gargova, S. A., Koleva, L. A., & Angelov, A. I. (2005). Aspergillus niger phytase: Purification and characterization. Biotechnology and Biotechnological Equipment, 19, 98–105.
Sato, K., Hamada, M., Asai, K., & Mituyama, T. (2009). Centroidfold: A web server for RNA secondary structure prediction. Nucleic Acids Research, 37, 277–280.
De Schutter, K., Lin, Y. C., Tiels, P., Van Hecke, A., Glinka, S., Weber-Lehmann, J., et al. (2009). Genome sequence of the recombinant protein production host Pichia pastoris. Nature Biotechnology, 27, 561–566.
Sebastian, S., Touchburn, S., Chavez, E., & Lague, P. (1996). Efficacy of supplemental microbial phytase at different dietary calcium levels on growth performance and mineral utilization of broiler chickens. Poultry Science, 75, 1516–1523.
Selle, P. H., & Ravindran, V. (2007). Microbial phytase in poultry nutrition. Animal Feed Science and Technology, 135, 1–41.
Sharp, P. M., & Li, W. H. (1987). The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Research, 15, 1281–1295.
Sinclair, G., & Choy, F. Y. M. (2002). Synonymous codon usage bias and the expression of human glucocerebrosidase in the methylotrophic yeast, Pichia pastoris. Protein Expression and Purification, 26, 96–105.
Singh, B., & Satyanarayana, T. (2006). A marked enhancement in phytase production by a thermophilic mould Sporotrichum thermophile using statistical designs in a cost-effective cane molasses medium. Journal of Applied Microbiology, 101, 344–352.
Singh, B., & Satyanarayana, T. (2006). Phytase production by a thermophilic mold Sporotrichum thermophile in solid state fermentation and its application in dephytinization of sesame oil cake. Applied Biochemistry and Biotechnology, 133, 239–250.
Singh, B., & Satyanarayana, T. (2008). Improved phytase production by a thermophilic mould Sporotrichum thermophile in submerged fermentation due to statistical designs. Bioresource Technology, 99, 824–830.
Singh, B., & Satyanarayana, T. (2008). Phytase production by a thermophilic mould Sporotrichum thermophile in solid state fermentation and its potential applications. Bioresource Technology, 99, 2824–2830.
Singh, B., & Satyanarayana, T. (2008). Phytase production by a thermophilic mould Sporotrichum thermophile in cost-effective cane molasses medium and its application in bread. Journal of Applied Microbiology, 105, 1858–1865.
Singh, B., & Satyanarayana, T. (2009). Characterization of a HAP-phytase from a thermophilic mould Sporotrichum thermophile. Bioresource Technology, 100, 2046–2051.
Sreekrishna, K., et al. (1997). Strategies for optimal synthesis and secretion of heterologous proteins in the methylotrophic yeast Pichia pastoris. Gene, 190, 55–62.
Tuller, T., Waldman, Y. Y., Kupiec, M., & Ruppin, E. (2010). Translation efficiency is determined by both codon bias and folding energy. PNAS USA, 107, 3645–3650.
Ullah, A. H. J., & Cummins, B. J. (1987). Purification, N-terminal amino acid sequence and characterisation of pH 2.5 optimum acid phosphatase (E.C.3.1.3.2) from Aspergillus ficuum. Preparative Biochemistry, 17, 397–422.
Ushasree, M. V., Vidya, J., & Pandey, A. (2014). Extracellular expression of a thermostable phytase (phyA) in Kluyveromyces lactis. Process Biochemistry, 49, 1440–1447.
Vats, P., & Banerjee, U. C. (2005). Biochemical characterisation of extracellular phytase (myo inositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. Journal of Industrial Microbiology and Biotechnology, 32, 141–147.
Villatte, F., Hussein, A. S., Bachmann, T. T., & Schmid, R. D. (2001). Expression level of heterologous proteins in Pichia pastoris is influenced by flask design. Applied Microbiology and Biotechnology, 55, 463–465.
Vohra, A., & Satyanarayana, T. (2003). Phytases: Microbial sources, production, purification, and potential biotechnological applications. Critical Reviews in Biotechnology, 23, 29–60.
Wang, Y., Gao, X., Su, Q., Wu, W., & An, L. (2007). Cloning, expression, and enzyme characterization of an acid heat-stable phytase from Aspergillus fumigatus WY-2. Current Microbiology, 55, 65–70.
Woo, J. H., et al. (2002). Gene optimization is necessary to express a bivalent anti-humananti-T cell immunotoxin in Pichia pastoris. Protein Expression and Purification, 25, 270–282.
Wyss, M., Pasamontes, L., Remy, R., Kohler, J., Kusznir, E., Gradient, M., et al. (1998). Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus phytase, A. niger phytase, and A. niger pH 2.5 acid phosphatase. Applied and Environmental Microbiology, 64, 4446–4451.
Acknowledgments
The authors are grateful to the Department of Biotechnology and University Grant Commission, Govt. of India, New Delhi, for financial assistance and fellowship during the course of this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ranjan, B., Satyanarayana, T. Recombinant HAP Phytase of the Thermophilic Mold Sporotrichum thermophile: Expression of the Codon-Optimized Phytase Gene in Pichia pastoris and Applications. Mol Biotechnol 58, 137–147 (2016). https://doi.org/10.1007/s12033-015-9909-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-015-9909-7