Abstract
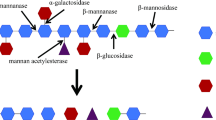
The purified extracellular xylanase of polyextremophilic Bacillus halodurans TSEV1 has been visualized as a single band on SDS-PAGE and eluted as single peak by gel filtration, with a molecular mass of 40 kDa. The peptide finger print and cloned xylanase gene sequence analyses indicate that this enzyme belongs to GH family 10. The active site carboxyl residues are mainly involved in catalysis, while tryptophan residues are involved in substrate binding. The enzyme is optimally active at 80 °C and pH 9.0, and stable in the pH range of 7.0–12.0 with T 1/2 of 35 min at 80 °C (pH 9.0). Activation energy for birch wood xylan hydrolysis is 30.51 kJ mol−1. The K m, V max and k cat (birchwood xylan) are 2.05 mg ml−1, 333.33 μmol mg−1 min−1 and 3.33 × 104 min−1, respectively. The pKa1 and pKa2 of ionizable groups of the active site that influence V max are 8.51 and 11.0. The analysis of thermodynamic parameters for xylan hydrolysis suggests this as a spontaneous process. The enzyme is resistant to chemical denaturants like urea and guanidinium-HCl. The site-directed mutagenesis of catalytic glutamic acid residues (E196 and E301) resulted in a complete loss of activity. The birch wood xylan hydrolyzate contained xylobiose and xylotriose as the main products without any trace of xylose, and the enzyme hydrolyzes xylotetraose and xylopentaose rapidly to xylobiose. Thermo-alkali-stability, resistance to various chemical denaturants and mode of action make it a useful biocatalyst for generating xylo-oligosaccharides from agro-residues and bleaching of pulp in paper industries.







Similar content being viewed by others
References
Aachary AA, Prapulla SG (2009) Value addition to corncob: production and characterization of xylooligosaccharides from alkali pre-treated lignin-saccharide complex using Aspergillus oryzae MTCC 5154. Biores Technol 100:991–995
Adsul MG, Bastawde KB, Gokhale DV (2009) Biochemical characterization of two xylanases from yeast Pseudozyma hubeiensis producing only xylooligosaccharides. Biores Technol 100:6488–6495
Anand A, Kumar V, Satyanarayana T (2013) Characteristics of thermostable endoxylanase and β-xylosidase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1 and its applicability in generating xylooligosaccharides and xylose from agro-residues. Extremophiles 17:357–366
Bazzicalupo M, Fani R (1994) The use of RAPD for generating specific DNA probes for microorganisms. In: Clap JP (ed) Methods in molecular biology, species diagnostic protocols: PCR and other nucleic acid methods. Humana Press Inc, Totowa NJ, pp 155–175
Bokhari SAI, Latif F, Rajoka MI (2009) Purification and characterization of xylanases from Thermomyces lanuginosus and its mutant derivative possessing novel kinetic and thermodynamic properties. World J Microbiol Biotechnol 25:493–502
Campbell JM, Fahey GC, Wolf BW (1997) Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127:130–136
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Dixon M, Webb EC (1979) Enzyme kinetics. In: Dixon M, Webb EC (eds) Enzymes, vol 3. Academic Press, New York, pp 47–206
Dodia MS, Bhimani HG, Rawal CM, Joshi RH, Singh SP (2008) Salt dependent resistance against chemical denaturation of alkaline protease from a newly isolated haloalkaliphilic Bacillus sp. Biores Technol 99:6223–6227
Eyring H, Stearn AE (1939) The application of the theory of absolute reaction rates to proteins. Chem Rev 24:253–270
Georis J, Esteves FD, Lamotte-Brasseur J, Bougnet V, Devreese B, Giannotta F, Granier B, Frere JM (2000) An additional aromatic interaction improves the thermostability and thermophilicity of a mesophilic family 11 xylanase: structural basis and molecular study. Protein Sci 9:466–475
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Hsu CK, Liao JW, Chung YC, Hsieh CP, Chan YC (2004) Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr 134:1523–1528
Kar S, Sona GS, Das A, Jana A, Maity C, Mandal A, Das Mohapatra P, Pati B, Mondal K (2012) Process optimization of xylanase production using cheap solid substrate by Trichoderma reesei SAF3 and study on the alteration of behavioural properties of enzyme obtained from SSF and SmF. Bioprocess Biosyst Eng 36:1–12
Klibanov AM (1983) Stabilization of enzymes against thermal inactivation. In: Allen IL (ed) Advances in applied microbiology, vol, 29. pp 1–28
Kumar V, Satyanarayana T (2011) Applicability of thermo-alkali-stable and cellulase-free xylanase from a novel thermo-halo-alkaliphilic Bacillus halodurans in producing xylooligosaccharides. Biotechnol Lett 33:2279–2285
Kumar V, Satyanarayana T (2012) Thermo-alkali-stable xylanase of a novel polyextremophilic Bacillus halodurans TSEV1 and its application in biobleaching. Intern Biodet Biodegrad 75:138–145
Kumar V, Syal P, Satyanarayana T (2012) Highly thermo–halo–alkali-stable β-1,4-endoxylanase from a novel polyextremophilic strain of Bacillus halodurans. Bioprocess Biosyst Eng 36:555–565
Kyu KL, Ratanakhanokchai K, Uttapap D, Tanticharoen M (1994) Induction of xylanase in Bacillus circulans B6. Biores Technol 48:163–167
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Mamo G, Hatti-Kaul R, Mattiasson B (2007) A thermostable alkaline active endo-β-1-4-xylanase from Bacillus halodurans S7: purification and characterization. Enz Microb Technol 39:1492–1498
Mishra M, Thakur IS (2011) Purification, characterization and mass spectroscopic analysis of thermo-alkalotolerant β-1, 4 endoxylanase from Bacillus sp. and its potential for dye decolorization. Inter Biodet Biodegrad 65:301–308
Mohana S, Shah A, Divecha J, Madamwar D (2008) Xylanase production by Burkholderia sp. DMAX strain under solid state fermentation using distillery spent wash. Biores Technol 99:7553–7564
Montes FJ, Battaner E, Catalán J, Galán Ma (1995) Kinetics and heat-inactivation mechanisms of d-amino acid oxidase. Process Biochem 30:217–224
Mussatto SI, Mancilha IM (2007) Non-digestible oligosaccharides: a review. Carbohydr Polym 68:587–597
Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591
Rashid MH, Siddiqui KS (1998) Thermodynamic and kinetic study of stability of the native and chemically modified β-glucosidases from Aspergillus niger. Process Biochem 33:109–115
Ratanakhanokchai K, Kyu KL, Tanticharoen M (1999) Purification and properties of a xylan-binding endoxylanase from alkaliphilic Bacillus sp. strain K-1. Appl Environ Microbiol 65:694–697
Saqib AAN, Hassan M, Khan NF, Baig S (2010) Thermostability of crude endoglucanase from Aspergillus fumigatus grown under solid state fermentation (SSF) and submerged fermentation (SmF). Process Biochem 45:641–646
Satyanarayana T, Sharma A, Mehta D, Puri A, Kumar V, Nisha M, Joshi S (2012) Biotechnological applications of biocatalysts from the firmicutes Bacillus and Geobacillus species. In: Satyanarayana T, Johri BN (eds) Microorganisms in sustainable agriculture and biotechnology. Springer, Netherlands, pp 343–379
Siddiqui KS, Azhar MJ, Rashid MH, Rajoka MI (1996) Activity and thermostability of carboxymethylcellulase from Aspergillus niger is strongly influenced by non-covalently attached polysaccharides. World J Microbiol Biotechnol 12:213–216
Srinivasan MC, Rele MV (1999) Microbial xylanases for paper industry. Curr Sci 77:137–142
Techapun C, Poosaran N, Watanabe M, Sasaki K (2003) Thermostable and alkaline-tolerant microbial cellulase-free xylanases produced from agricultural wastes and the properties required for use in pulp bleaching bioprocesses: a review. Process Biochem 38:1327–1340
Torronen A, Rouvinen J (1995) Structural comparison of two major endo-1,4-xylanases from Trichoderma reesei. Biochemistry 34:847–856
Vafiadi C, Christakopoulos P, Topakas E (2010) Purification, characterization and mass spectrometric identification of two thermophilic xylanases from Sporotrichum thermophile. Process Biochem 45:419–424
Vázquez MJ, Alonso JL, Domínguez H, Parajó JC (2000) Xylooligosaccharides: manufacture and applications. Trends Food Sci Tec 11:387–393
Vieille C, Zeikus GJ (1996) Thermozymes: identifying molecular determinants of protein structural and functional stability. Trends Biotechnol 14:183–190
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43
Wong KKY, Nelson SL, Saddler JN (1996) Xylanase treatment for the peroxide bleaching of oxygen delignified kraft pulps derived from three softwood species. J Biotechnol 48:137–145
Xiong HR, Nyyssola A, Janis J, Pastinen O, von Weymarn N, Leisola M, Turunen O (2004) Characterization of the xylanase produced by submerged cultivation of Thermomyces lanuginosus DSM 10635. Enz Microb Technol 35(1):93–99
Zamost B, Nielsen H, Starnes R (1991) Thermostable enzymes for industrial applications. J Ind Microbiol 8(2):71–81
Acknowledgments
Authors are grateful to the Ministry of Environment & Forests, Department of Biotechnology, and Indian Council of Medical Research, Government of India, New Delhi for financial assistance while carrying out this investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Driessen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, V., Satyanarayana, T. Biochemical and thermodynamic characteristics of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 17, 797–808 (2013). https://doi.org/10.1007/s00792-013-0565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0565-1