Abstract
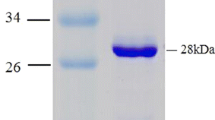
Replacement of chemical steps with biocatalytic ones is becoming increasingly more interesting due to the remarkable catalytic properties of enzymes, such as their wide range of substrate specificities and variety of chemo-, stereo- and regioselective reactions. This study presents characterisation of an alcohol dehydrogenase (ADH) from the halophilic archaeum Halobacterium sp. NRC-1 (HsADH2). A hexahistidine-tagged recombinant version of HsADH2 (His-HsADH2) was heterologously overexpressed in Haloferax volcanii. The enzyme was purified in one step by immobilised Ni-affinity chromatography. His-HsADH2 was halophilic and mildly thermophilic with optimal activity for ethanol oxidation at 4 M KCl around 60 °C and pH 10.0. The enzyme was extremely stable, retaining 80 % activity after 30 days. His-HsADH2 showed preference for NADP(H) but interestingly retained 60 % activity towards NADH. The enzyme displayed broad substrate specificity, with maximum activity obtained for 1-propanol. The enzyme also accepted secondary alcohols such as 2-butanol and even 1-phenylethanol. In the reductive reaction, working conditions for His-HsADH2 were optimised for acetaldehyde and found to be 4 M KCl and pH 6.0. His-HsADH2 displayed intrinsic organic solvent tolerance, which is highly relevant for biotechnological applications.


Similar content being viewed by others
References
Schmid, A., Dordick, J. S., Hauer, B., Kiener, A., Wubbolts, M., & Witholt, B. (2001). Industrial biocatalysis today and tomorrow. Nature, 409, 258–268.
Schoemaker, H. E., Mink, D., & Wubbolts, M. G. (2003). Dispelling the myths—biocatalysis in industrial synthesis. Science, 299, 1694–1697.
Kroutil, W., Mang, H., Edegger, K., & Faber, K. (2004). Recent advances in the biocatalytic reduction of ketones and oxidation of sec-alcohols. Current Opinion in Chemical Biology, 8, 120–126.
Yakushi, T., & Matsushita, K. (2010). Alcohol dehydrogenase of acetic acid bacteria: Structure, mode of action, and applications in biotechnology. Applied Microbiology and Biotechnology, 86, 1257–1265.
Gargiulo, S., Arends, I., & Hollmann, F. (2011). A photoenzymatic system for alcohol oxidation. ChemCatChem, 3, 338–342.
Klibanov, A. M. (2003). Asymmetric enzymatic oxidoreductions in organic solvents. Current Opinion in Biotechnology, 14, 427–431.
Klibanov, A. M. (2001). Improving enzymes by using them in organic solvents. Nature, 409, 241–246.
Cainelli, G., Engel, P. C., Galletti, P., Giacomini, D., Gualandi, A., & Paradisi, F. (2005). Engineered phenylalanine dehydrogenase in organic solvents: Homogeneous and biphasic enzymatic reactions. Organic & Biomolecular Chemistry, 3, 4316–4320.
Danson, M. J., & Hough, D. W. (1997). The structural basis of protein halophilicity. Comparative Biochemistry and Physiology Part A, Physiology, 117, 307–312.
Lanyi, J. K. (1974). Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriological Reviews, 38, 272–290.
Timpson, L., Alsafadi, D., Mac Donnchadha, C., Liddell, S., Sharkey, M., & Paradisi, F. (2012). Characterization of alcohol dehydrogenase (ADH12) from Haloarcula marismortui, an extreme halophile from the Dead Sea. Extremophiles, 16, 57–66.
Timpson, L., Liliensiek, A.-K., Alsafadi, D., Cassidy, J., Sharkey, M., Liddell, S., et al. (2012). A comparison of two novel alcohol dehydrogenase enzymes (ADH1 and ADH2) from the extreme halophile Haloferax volcanii. Applied Microbiology and Biotechnology, 96(6), 1–9.
Alsafadi, D., & Paradisi, F. (2013). Effect of organic solvents on the activity and stability of halophilic alcohol dehydrogenase (ADH2) from Haloferax volcanii. Extremophiles, 44(2), 511–518.
Dyall-Smith, M. (2008). The halohandbook: Protocols for halobacterial genetics (Vol. 7). Boca Raton: CRC Press.
Allers, T., Barak, S., Liddell, S., Wardell, K., & Mevarech, M. (2010). Improved strains and plasmid vectors for conditional overexpression of His-tagged proteins in Haloferax volcanii. Applied and Environmental Microbiology, 76, 1759–1769.
Wilkinson, G. N. (1961). Statistical estimations in enzyme kinetics. The Biochemical Journal, 80, 324–332.
Ng, W. V., Kennedy, S. P., Mahairas, G. G., Berquist, B., Pan, M., Shukla, H. D., et al. (2000). Genome sequence of Halobacterium species NRC-1. Proceedings of the National Academy of Sciences of the United States of America, 97, 12176–12181.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
Terpe, K. (2003). Overview of tag protein fusions: From molecular and biochemical fundamentals to commercial systems. Applied Microbiology and Biotechnology, 60, 523–533.
Woodyer, R., van der Donk, W. A., & Zhao, H. (2003). Relaxing the nicotinamide cofactor specificity of phosphite dehydrogenase by rational design. Biochemistry (Mosc)., 42, 11604–11614.
Oren, A. (1983). A thermophilic amyloglucosidase from. Halobacterium sodomense, a halophilic bacterium from the Dead Sea. Current Microbiology, 8, 225–230.
Bonete, M. J., Camacho, M. L., & Cadenas, E. (1987). A new glutamate dehydrogenase from Halobacterium halobium with different coenzyme specificity. International Journal of Biochemistry, 19, 1149–1155.
Bonete, J. M., Camacho, M. L., & Cadenas, E. (1986). Purification and some properties of NAD +-dependent glutamate dehydrogenase from Halobacterium halobium. International Journal of Biochemistry, 18, 785–789.
Acknowledgments
FP acknowledges funding under the Science Foundation Ireland Research Framework Programme and the EPA. Kind donations of the strain H. sp. NRC-1 by Prof. P Engel and the expression system H. volcanii (H1209-H1325)/-pTA963 by Dr. T. Allers are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ann-Kathrin Liliensiek and Jennifer Cassidy contributed equally to this study.
Rights and permissions
About this article
Cite this article
Liliensiek, AK., Cassidy, J., Gucciardo, G. et al. Heterologous Overexpression, Purification and Characterisation of an Alcohol Dehydrogenase (ADH2) from Halobacterium sp. NRC-1. Mol Biotechnol 55, 143–149 (2013). https://doi.org/10.1007/s12033-013-9666-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-013-9666-4