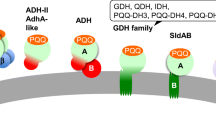
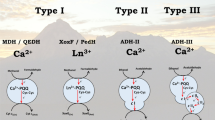
Abstract
Pyrroquinoline quinone-dependent alcohol dehydrogenase (PQQ-ADH) of acetic acid bacteria is a membrane-bound enzyme involved in the acetic acid fermentation by oxidizing ethanol to acetaldehyde coupling with reduction of membranous ubiquinone (Q), which is, in turn, re-oxidized by ubiquinol oxidase, reducing oxygen to water. PQQ-ADHs seem to have co-evolved with the organisms fitting to their own habitats. The enzyme consists of three subunits and has a pyrroloquinoline quinone, 4 heme c moieties, and a tightly bound Q as the electron transfer mediators. Biochemical, genetic, and electrochemical studies have revealed the unique properties of PQQ-ADH since it was purified in 1978. The enzyme is unique to have ubiquinol oxidation activity in addition to Q reduction. This mini-review focuses on the molecular properties of PQQ-ADH, such as the roles of the subunits and the cofactors, particularly in intramolecular electron transport of the enzyme from ethanol to Q. Also, we summarize biotechnological applications of PQQ-ADH as to enantiospecific oxidations for production of the valuable chemicals and bioelectrocatalysis for sensors and fuel cells using indirect and direct electron transfer technologies and discuss unsolved issues and future prospects related to this elaborate enzyme.





Similar content being viewed by others
References
Adachi O, Miyagawa E, Shinagawa E, Matsushita K, Ameyama M (1978a) Purification and properties of particulate alcohol dehydrogenase from Acetobacter aceti. Agric Biol Chem 42(12):2341–2346
Adachi O, Tayama K, Shinagawa E, Matsushita K, Ameyama M (1978b) Purification and characterization of particulate alcohol dehydrogenase from Gluconobacter suboxydans. Agric Biol Chem 42(11):2045–2056
Ameyama M, Matsushita K, Shinagawa E, Adachi O (1987) Sugar-oxidizing respiratory chain of Gluconobacter suboxydans. Evidence for a branched respiratory chain and characterization of respiratory chain-linked cytochromes. Agric Biol Chem 51(11):2943–2950
Azuma Y, Hosoyama A, Matsutani M, Furuya N, Horikawa H, Harada T, Hirakawa H, Kuhara S, Matsushita K, Fujita N, Shirai M (2009) Whole-genome analyses reveal genetic instability of Acetobacter pasteurianus. Nucleic Acids Res 37(17):5768–5783
Bader MW, Xie T, Yu CA, Bardwell JC (2000) Disulfide bonds are generated by quinone reduction. J Biol Chem 275(34):26082–26088
Cunningham L, Pitt M, Williams HD (1997) The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol Microbiol 24(3):579–591
Duine JA, Frank J, De Beer R (1984) An electron-nuclear double-resonance study of methanol dehydrogenase and its coenzyme radical. Arch Biochem Biophys 233(2):708–711
Elias MD, Nakamura S, Migita CT, Miyoshi H, Toyama H, Matsushita K, Adachi O, Yamada M (2004) Occurrence of a bound ubiquinone and its function in Escherichia coli membrane-bound quinoprotein glucose dehydrogenase. J Biol Chem 279(4):3078–3083
Frébortová J, Matsushita K, Yakushi T, Toyama H, Adachi O (1997) Quinoprotein alcohol dehydrogenase of acetic acid bacteria: Kinetic study on the enzyme purified from Acetobacter methanolicus. Biosci Biotechnol Biochem 61(3):459–465
Frébortová J, Matsushita K, Arata H, Adachi O (1998) Intramolecular electron transport in quinoprotein alcohol dehydrogenase of Acetobacter methanolicus: a redox-titration study. Biochim Biophys Acta 1363(1):24–34
Geerlof A, van Tol JBA, Jongejan JA, Duine JA (1994) Enantioselective conversions of the racemic C3-alcohol synthons, glycidol (2, 3-epoxy-1-propanol), and solketal (2, 2-dimethyl-4-(hydroxymethyl)-1, 3-dioxolane) by quinohaemoprotein alcohol dehydrogenases and bacteria containing such enzymes. Bioscience, Biotechnology and Biochemistry 42(1):8–15
Gómez-Manzo S, Contreras-Zentella M, González-Valdez A, Sosa-Torres M, Arreguín-Espinoza R, Escamilla-Marván E (2008) The PQQ-alcohol dehydrogenase of Gluconacetobacter diazotrophicus. Int J Food Microbiol 125(1):71–78
Habe H, Fukuoka T, Kitamoto D, Sakaki K (2009a) Biotransformation of glycerol to D-glyceric acid by Acetobacter tropicalis. Appl Microbiol Biotechnol 81(6):1033–1039
Habe H, Shimada Y, Yakushi T, Hattori H, Ano Y, Fukuoka T, Kitamoto D, Itagaki M, Watanabe K, Yanagishita H, Matsushita K, Sakaki K (2009b) Microbial production of glyceric acid, an organic acid that can be mass produced from glycerol. Appl Environ Microbiol 75(24):7760–7766
Ikeda T, Kano K (2003) Bioelectrocatalysis-based application of quinoproteins and quinoprotein-containing bacterial cells in biosensors and biofuel cells. Biochim Biophys Acta 1647(1–2):121–126
Ikeda T, Kobayashi D, Matsushita F (1993) Bioelectrocatalysis at electrodes coated with alcohol dehydrogenase, a quinohemoprotein with heme c serving as a built-in mediator. J Electroanal Chem 361:221–228
Inoue T, Sunagawa M, Mori A, Imai C, Fukuda M, Takagi M, Yano K (1989) Cloning and sequencing of the gene encoding the 72-kilodalton dehydrogenase subunit of alcohol dehydrogenase from Acetobacter aceti. J Bacteriol 171(6):3115–3122
Inoue T, Sunagawa M, Mori A, Imai C, Fukuda M, Takagi M, Yano K (1992) Cloning and sequencing of the gene encoding the 45-kilodalton subunit of alcohol dehydrogenase from Acetobacter aceti. J Ferment Bioeng 73(6):419–424
Kamitaka Y, Tsujimura S, Setoyama N, Kajino T, Kano K (2007) Fructose/dioxygen biofuel cell based on direct electron transfer-type bioelectrocatalysis. Phys Chem Chem Phys 9(15):1793–1801
Kanchanarach W, Theeragool G, Yakushi T, Toyama H, Adachi O, Matsushita K (2009) Characterization of thermotolerant Acetobacter pasteurianus strains and their quinoprotein alcohol dehydrogenases. Appl Microbiol Biotechnol 85(3):741–751
Kondo K, Beppu T, Horinouchi S (1995) Cloning, sequencing, and characterization of the gene encoding the smallest subunit of the three-component membrane-bound alcohol dehydrogenase from Acetobacter pasteurianus. J Bacteriol 177(17):5048–5055
Machado SS, Wandel U, Jongejan JA, Straathof AJJ, Duine JA (1999) Characterization of the enantioselective properties of the quinohemoprotein alcohol dehydrogenase of Acetobacter pasteurianus LMG 1635. 1. Different enantiomeric ratios of whole cells and purified enzyme in the kinetic resolution of racemic glycidol. Biosci Biotechnol Biochem 63(1):10–20
Masud U, Matsushita K, Theeragool G (2010) Cloning and functional analysis of adhS gene encoding quinoprotein alcohol dehydrogenase subunit III from Acetobacter pasteurianus SKU1108. Int J Food Microbiol 138(1–2):39–49
Matsushita K, Nagatani Y, Shinagawa E, Adachi O, Ameyama M (1989) Effect of extracellular pH on the respiratory chain and energetics of Gluconobacter suboxydans. Agric Biol Chem 53(11):2895–2902
Matsushita K, Nagatani Y, Shinagawa E, Adachi O, Ameyama M (1991) Reconstitution of the ethanol oxidase respiratory chain in membranes of quinoprotein alcohol dehydrogenase-deficient Gluconobacter suboxydans subsp. alpha strains. J Bacteriol 173(11):3440–3445
Matsushita K, Takaki Y, Shinagawa E, Ameyama M, Adachi O (1992) Ethanol oxidase respiratory chain of acetic acid bacteria. Reactivity with ubiquinone of pyrroloquinoline quinone-dependent alcohol dehydrogenases purified from Acetobacter aceti and Gluconobacter suboxydans. Biosci Biotechnol Biochem 56(2):304–310
Matsushita K, Toyama H, Adachi O (1994) Respiratory chains and bioenergetics of acetic acid bacteria. In: Rose AH, Tempest DW (eds) Advances in microbial physiology, vol 36. Academic, London, pp 247–301
Matsushita K, Yakushi T, Takaki Y, Toyama H, Adachi O (1995) Generation mechanism and purification of an inactive form convertible in vivo to the active form of quinoprotein alcohol dehydrogenase in Gluconobacter suboxydans. J Bacteriol 177(22):6552–6559
Matsushita K, Yakushi T, Toyama H, Shinagawa E, Adachi O (1996) Function of multiple heme c moieties in intramolecular electron transport and ubiquinone reduction in the quinohemoprotein alcohol dehydrogenase-cytochrome c complex of Gluconobacter suboxydans. J Biol Chem 271(9):4850–4857
Matsushita K, Yakushi T, Toyama H, Adachi O, Miyoshi H, Tagami E, Sakamoto K (1999) The quinohemoprotein alcohol dehydrogenase of Gluconobacter suboxydans has ubiquinol oxidation activity at a site different from the ubiquinone reduction site. Biochim Biophys Acta 1409(3):154–164
Matsushita K, Toyama H, Adachi O (2004) Respiratory chains in acetic acid bacteria: Membrane-bound periplasmic sugar and alcohol respirations. In: Zannoni D (ed) Respiration in Archaea and Bacteria. Springer, Dordrecht, pp 81–99
Matsushita K, Kobayashi Y, Mizuguchi M, Toyama H, Adachi O, Sakamoto K, Miyoshi H (2008) A tightly bound quinone functions in the ubiquinone reaction sites of quinoprotein alcohol dehydrogenase of an acetic acid bacterium, Gluconobacter suboxydans. Biosci Biotechnol Biochem 72(10):2723–2731
Mitsukura K, Uno T, Yoshida T, Nagasawa T (2007) Microbial asymmetric oxidation of 2-butyl-1, 3-propanediol. Appl Microbiol Biotechnol 76(1):61–65
Mogi T, Ano Y, Nakatsuka T, Toyama H, Muroi A, Miyoshi H, Migita CT, Ui H, Shiomi K, Omura S, Kita K, Matsushita K (2009) Biochemical and spectroscopic properties of cyanide-insensitive quinol oxidase from Gluconobacter oxydans. J Biochem 146(2):263–271
Molinari F, Villa R, Aragozzini F, Leon R, Prazeres DMF (1999) Enantioselective oxidation of (RS)-2-phenyl-1-propanol to (S)-2-phenylpropanoic acid with Gluconobacter oxydans: Simplex optimization of the biotransformation. Tetrahedron Asymmetry 10(15):3003–3009
Molinari F, Gandolfi R, Villa R, Urban E, Kiener A (2003) Enantioselective oxidation of prochiral 2-methyl-1,3-propandiol by Acetobacter pasteurianus. Tetrahedron Asymmetry 14(14):2041–2043
Niculescu M, Erichsen T, Sukharev V, Kerenyi Z, Csöregi E, Schuhmann W (2002) Quinohemoprotein alcohol dehydrogenase-based reagentless amperometric biosensor for ethanol monitoring during wine fermentation. Analytica Chimica Acta 463(1):39–51
Ohta H, Tetsukawa H, Noto N (1982) Enantiotopically selective oxidation of α, ω-diols with the enzyme systems of microorganisms. Journal of Organic Chemistry 47(12):2400–2404
Pugsley AP (1993) The complete general secretory pathway in gram-negative bacteria. Microbiol Rev 57(1):50–108
Ruzicka FJ, Beinert H, Schepler KL, Dunham WR, Sands RH (1975) Interaction of ubisemiquinone with a paramagnetic component in heart tissue. Proc Natl Acad Sci USA 72(8):2886–2890
Sato-Watanabe M, Mogi T, Ogura T, Kitagawa T, Miyoshi H, Iwamura H, Anraku Y (1994) Identification of a novel quinone-binding site in the cytochrome bo complex from Escherichia coli. J Biol Chem 269(46):28908–28912
Shinagawa E, Matsushita K, Adachi O, Ameyama M (1989) Formation of the apo-form of quinoprotein alcohol dehydrogenase from Gluconobacter suboxydans. Agric Biol Chem 53(7):1823–1828
Shinagawa E, Toyama H, Matsushita K, Tuitemwong P, Theeragool G, Adachi O (2006) A novel type of formaldehyde-oxidizing enzyme from the membrane of Acetobacter sp. SKU 14. Biosci Biotechnol Biochem 70(4):850–857
Tamaki T, Fukaya M, Takemura H, Tayama K, Okumura H, Kawamura Y, Nishiyama M, Horinouchi S, Beppu T (1991) Cloning and sequencing of the gene cluster encoding two subunits of membrane-bound alcohol dehydrogenase from Acetobacter polyoxogenes. Biochim Biophys Acta 1088(2):292–300
Tayama K, Fukaya M, Okumura H, Kawamura Y, Beppu T (1989) Purification and characterization of membrane-bound alcohol dehydrogenase from Acetobacter polyoxogenes sp. nov. Appl Microbiol Biotechnol 32(2):181–185
Tkac J, Svitel J, Vostiar I, Navratil M, Gemeiner P (2009) Membrane-bound dehydrogenases from Gluconobacter sp.: interfacial electrochemistry and direct bioelectrocatalysis. Bioelectrochemistry 76(1–2):53–62
Trcek J, Toyama H, Czuba J, Misiewicz A, Matsushita K (2006) Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Appl Microbiol Biotechnol 70(3):366–373
Yamada Y, Yukphan P (2008) Genera and species in acetic acid bacteria. Int J Food Microbiol 125(1):15–24
Yamashita T, Nakamaru-Ogiso E, Miyoshi H, Matsuno-Yagi A, Yagi T (2007) Roles of bound quinone in the single subunit NADH-quinone oxidoreductase (Ndi1) from Saccharomyces cerevisiae. J Biol Chem 282(9):6012–6020
Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S (2003) Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299(5607):700–704
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakushi, T., Matsushita, K. Alcohol dehydrogenase of acetic acid bacteria: structure, mode of action, and applications in biotechnology. Appl Microbiol Biotechnol 86, 1257–1265 (2010). https://doi.org/10.1007/s00253-010-2529-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2529-z