Abstract
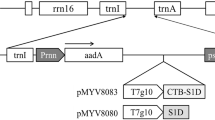
The synthetic cholera toxin B subunit (CTB) gene, modified according to the optimized codon usage of plant genes, was introduced into a plant expression vector and expressed under the control of the Bx17 HMW (high molecular weight) wheat endosperm-specific promoter containing an intron of the rice act1. The recombinant vector was transformed into rice plants using a biolistic-mediated transformation method. Stable integration of the synthetic CTB gene into the chromosomal DNA was confirmed by PCR amplification analysis. A high level of CTB (2.1% of total soluble protein) was expressed in the endosperm tissue of the transgenic rice plants. The synthetic CTB produced only in the rice endosperm demonstrated strong affinity for GM1-ganglioside, thereby suggesting that the CTB subunits formed an active pentamer. The successful expression of CTB genes in transgenic plants makes it a powerful tool for the development of a plant-derived edible vaccine.




Similar content being viewed by others
References
Daniell, H., Streatfield, S. J., & Wycoff, K. (2001). Medical molecular farming: Production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends in Plant Science, 6, 219–226. doi:10.1016/S1360-1385(01)01922-7.
Mason, H. S., Warzecha, H., Mor, T., & Arntzen, C. J. (2002). Edible plant vaccines: Applications for prophylactic and therapeutic molecular medicine. Trends in Molecular Medicine, 8, 324–329. doi:10.1016/S1471-4914(02)02360-2.
Walmsley, A. M., & Arntzen, C. J. (2003). Plant cell factories and mucosal vaccines. Current Opinion in Biotechnology, 14, 145–150. doi:10.1016/S0958-1669(03)00026-0.
Faruque, S. M., Biswas, K., Udden, S. M., Ahmad, Q. S., Sack, D. A., Nair, G. B., et al. (2006). Transmissibility of cholera: In vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proceedings of the National Academy of Sciences of the United States of America, 103, 6350–6355. doi:10.1073/pnas.0601277103.
Kaper, J. B., Morris, G., & Levine, M. M. (1995). Cholera. Clinical Microbiology Reviews, 8, 316.
Giddings, G. (2001). Transgenic plants as protein factories. Current Opinion in Biotechnology, 12, 450–454. doi:10.1016/S0958-1669(00)00244-5.
Sixma, T. K., Pronk, S. E., Kalk, K. H., Wartna, E. S., van Zanten, B. A., Witholt, B., et al. (1991). Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature, 351, 371–377. doi:10.1038/351371a0.
Zhang, R. G., Scott, D. L., Westbrook, M. L., Nance, S., Spangler, B. D., Shipley, G. G., et al. (1995). The three-dimensional crystal-structure of cholera toxin. Journal of Molecular Biology, 251, 563–573. doi:10.1006/jmbi.1995.0456.
Holmgren, J., Adamsson, J., Anjuère, J., Clemens, J., Czerkinsky, C., Eriksson, K., et al. (2005). Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunology Letters, 97, 181–188. doi:10.1016/j.imlet.2004.11.009.
Field, M., Rao, M. C., & Chang, E. B. (1989). Intestinal electrolyte transport and diarrheal disease. The New England Journal of Medicine, 321, 800–806.
Mckenzie, S. J., & Halsey, J. F. (1984). Cholera toxin-B subunit as a carrier protein to stimulate a mucosal immune-response. Journal of Immunology (Baltimore, Md.: 1950), 133, 1818–1824.
Czerkinsky, C., Russell, M. W., Lycke, N., Linblad, M., & Holmgren, J. (1989). Oral-administration of a streptococcal antigen couples to cholera-toxin B-subunit evokes strong antibody-responses in salivary-glands and extramucosal tissues. Infection and Immunity, 57, 1072–1077.
Dertzbaugh, M. T., & Elson, C. O. (1993). Reduction in oral immunogenicity of cholera toxin-B subunit by N-terminal peptide addition. Infection and Immunity, 61, 384–390.
Elson, C. O. (1989). Cholera toxin and its subunits as potential oral adjutants. Current Topics in Microbiology and Immunology, 146, 29–33.
Merritt, E. A., Sarfaty, S., Feil, I. K., & Hol, W. G. J. (1997). Structural foundation for the design of receptor antagonists targeting Escherichia coli heat-labile enterotoxin. Structure (London, England), 5, 1485–1499. doi:10.1016/S0969-2126(97)00298-0.
Butow, B. J., Ma, W., Gale, K. R., Cornish, G. B., Rampling, L., Larroque, O., et al. (2003). Molecular discrimination of Bx7 alleles demonstrates that a highly expressed high-molecular-weight glutenin allele has a major impact on wheat flour dough strength. Theoretical and Applied Genetics, 107, 1524–1532. doi:10.1007/s00122-003-1396-8.
Butow, B. J., Gale, K. R., Ikea, J., Juhasz, A., Bedo, Z., Tamas, L., et al. (2004). Dissemination of the highly expressed Bx7 glutenin subunit (Glu-B1al allele) in wheat as revealed by novel PCR markers and RP-HPLC. Theoretical and Applied Genetics, 109, 1525–1535. doi:10.1007/s00122-004-1776-8.
Shewry, P. R., Tatham, A. S., & Halford, N. G. (1999). The prolamins of the Triticeae. In R. Casey & P. R. Shewry (Eds.), Seed proteins (pp. 35–78). Kluwer Academic Press: Doldrecht.
Lamacchia, C., Shewry, P. R., Di Fonzo, N., Forsyth, J. L., Harris, N., Lazzeri, P. A., et al. (2001). Endosperm-specific activity of a storage protein gene promoter in transgenic wheat seed. Journal of Experimental Botany, 52, 234–250. doi:10.1093/jexbot/52.355.243.
Oszvald, M., Kang, T. J., Jenes, B., Kim, T. G., Tamas, L., & Yang, M. S. (2007). Synthesis and assembly of Escherichia coli heat-labile enterotoxin B subunit in transgenic rice (Oryza sativa L.). Biotechnology and Bioprocessing Engineering, 12, 676–686.
McElroy, D., Blowers, A. D., Jenes, B., & Wu, R. (1991). Construction of expression vectors based on the rice actin 1 (Act1) 5’ region for use in monocot transformation. Molecular and General Genetics, 231, 150–160. doi:10.1007/BF00293832.
Li, Y. Z., Ma, H. M., Zhang, J. Z., Wang, Z. Y., & Hong, M. M. (1995). Effects of the first intron of rice waxy gene on the expression of foreign genes in rice and tobacco protoplasts. Plant Science, 108, 181–190. doi:10.1016/0168-9452(95)04143-I.
Oszvald, M., Gardonyi, M., Tamas, C., Takacs, I., Jenes, B., & Tamas, L. (2008). Development and characterization of a chimaeric tissue specific promoter in wheat and rice endosperm. In Vitro Cellular & Developmental Biology. Plant, 44, 1–7. doi:10.1007/s11627-007-9082-1.
Wang, X. G., Zhang, G. H., Liu, C. X., Zhang, Y. H., Xiao, C. Z., & Fang, R. X. (2001). Purified cholera toxin B subunit from transgenic tobacco plants possesses authentic antigenicity. Biotechnology and Bioengineering, 72, 490–494. doi:10.1002/1097-0290(20010220)72:4 ≤ 490::AID-BIT1011 ≥ 3.0.CO;2-0.
Li, D., O’Leary, J., Huang, Y., Huner, N. P. A., Jevnikar, A. M., & Ma, S. W. (2006). Expression of cholera toxin B subunit and the B chain of human insulin as a fusion protein in transgenic tobacco plants. Plant Cell Reports, 25, 417–424. doi:10.1007/s00299-005-0069-2.
Daniell, H., Lee, S. B., Panchal, T., & Wiebe, P. O. (2001). Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. Journal of Molecular Biology, 311, 1001–1009. doi:10.1006/jmbi.2001.4921.
Molina, A., Hervas-Stubbs, S., Daniell, H., Mingo-Castel, A. M., & Veramendi, J. (2004). High-yield expression of a viral peptide animal vaccine in transgenic tobacco chloroplasts. Plant Biotechnology Journal, 2, 141–153. doi:10.1046/j.1467-7652.2004.00057.x.
Arakawa, T., Chong, D. K., Merritt, J. L., & Langridge, W. H. (1997). Expression of cholera toxin B subunit oligomers in transgenic potato plants. Transgenic Research, 6, 403–413. doi:10.1023/A:1018487401810.
Kim, T. G., Galloway, D. R., & Langridge, W. H. R. (2004). Synthesis and assembly of anthrax lethal factor-cholera toxin B-subunit fusion protein in transgenic potato. Molecular Biotechnology, 28, 175–183. doi:10.1385/MB:28:3:175.
Choi, N. W., Estes, M. K., & Langridge, W. H. R. (2005). Synthesis and assembly of a cholera toxin B subunit-rotavirus VP7 fusion protein in transgenic potato. Molecular Biotechnology, 31, 193–2002. doi:10.1385/MB:31:3:193.
Jiang, X. L., He, Z. M., Peng, Z. Q., Qi, Y., Chen, Q., & Yu, S. Y. (2007). Cholera toxin B protein in transgenic tomato fruit induces systemic immune response in mice. Transgenic Research, 16, 169–175. doi:10.1007/s11248-006-9023-5.
Sharma, M. K., Singh, N. K., Jani, D., Sisodia, R., Thungapathra, M., Gautam, J. K., et al. (2008). Expression of toxin co-regulated piles subunit A (TCPA) of Vibrio cholera and its immunogenic epitopes fused to cholera toxin B subunit in transgenic tomato (Solanum lycopersicum). Plant Cell Reports, 27, 307–318. doi:10.1007/s00299-007-0464-y.
Rosales-Mendoza, S., Soria-Guerra, R. E., Olivera-Flores, M. T. D., Lopez-Revilla, R., Argullo-Astorga, G. R., & Jimenez-Bremont, J. F. (2007). Expression of Escherichia coli heat-labile enterotoxin b subunit (LTB) in carrot (Daucus carota L.). Plant Cell Reports, 26, 969–976. doi:10.1007/s00299-007-0310-2.
Kim, T. G., Kim, M. Y., Kim, B. G., Kang, T. J., Kim, Y. S., Jang, Y. S., et al. (2007). Synthesis and assembly of Escherichia coli heat-labile enterotoxin B subunit in transgenic lettuce (Lactuca sativa). Protein Expression and Purification, 51, 22–27. doi:10.1016/j.pep. 2006.05.024.
Athwal, D. S. (1971). Semidwarf rice and wheat in global food needs. The Quarterly Review of Biology, 4, 1–34. doi:10.1086/406754.
Pelham, H. R. B. (1988). Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. The EMBO Journal, 7, 913–918.
Kozak, M. (1989). The scanning model for translation: An update. The Journal of Cell Biology, 108, 229–241. doi:10.1083/jcb.108.2.229.
Kang, T. J., Loc, N. H., Jang, M. O., & Yang, M. S. (2004). Modification of the cholera toxin B subunit coding sequence to enhance expression in plants. Molecular Breeding, 13, 143–153. doi:10.1023/B:MOLB.0000018762.27841.7a.
Kang, T. J., Han, S. C., Jang, M. O., Kang, K. H., Jang, Y. S., & Yang, M. S. (2004). Enhanced expression of B-subunit of Escherichia coli heat-labile enterotoxin in tobacco by optimization of coding sequence. Applied Biochemistry and Biotechnology, 117, 175–187. doi:10.1385/ABAB:117:3:175.
Nagy, Z. B., Varga-Orvos, Z., Szakal, B., Tamas, L., & Puskas, L. G. (2006). Assembling and cloning genes for fusion proteins using reverse transcription one-step overlap extension PCR method. Analytical Biochemistry, 351, 311–313. doi:10.1016/j.ab.2006.01.049.
Chu, C. C., Wang, C. C., Sun, C. S., Hsu, C., Yin, K. C., & Chu, C. Y. (1975). Established an efficient medium for another culture of rice though competitive experiments on the nitrogen sources. Scientia Sinica, 18, 659–668.
Christou, P. (1997). Rice transformation: Bombardment. Plant Molecular Biology, 35, 197–203. doi:10.1023/A:1005791230345.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Plant Physiology, 15, 473–497. doi:10.1111/j.1399-3054.1962.tb08052.x.
Kang, T. J., & Yang, M. S. (2004). Rapid and reliable extraction of genomic DNA from various wild-type and transgenic plants. BMC Biotechnology, 4, 20. doi:10.1186/1472-6750-4-20.
Li, Z., & Trick, H. N. (2005). Rapid method for high-quality RNA isolation from seed endosperm containing high levels of starch. BioTechniques, 38, 872–876.
Dean, C., Jones, J., Favreau, M., Dunsmuir, P., & Bedbrook, J. (1988). Influence of flanking sequences on variability in expression levels of an introduced gene in transgenic tobacco plants. Nucleic Acids Research, 16, 9267–9283. doi:10.1093/nar/16.19.9267.
Peach, C., & Velten, J. (1991). Transgene expression variability position effect of CAT and GUS reporter genes driven by linked divergent T-DNA promoters. Plant Molecular Biology, 17, 49–60. doi:10.1007/BF00036805.
Schon, A., & Freire, E. (1989). Thermodynamics of intersubunit interactions in cholera toxin upon binding to the oligosaccharide portion of its cell surface receptor, ganglioside GM1. Biochemistry, 28, 5019–5024. doi:10.1021/bi00438a017.
Oszvald, M., Kang, T. J., Tomoskozi, S., Tamas, C., Tamas, L., Kim, T. G., et al. (2007). Expression of a synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat labile enterotoxin in rice endosperm. Molecular Biotechnology, 35, 215–223. doi:10.1007/BF02686007.
Jani, D., Meena, L. S., Rizwan-ul-Haq, Q. M., Singh, Y., Sharma, A. K., & Tyagi, A. K. (2002). Expression of cholera toxin B subunit in transgenic tomato plants. Transgenic Research, 11, 447–454. doi:10.1023/A:1020336332392.
Wiley, P. R., Tosi, P., Evrard, A., Lovegrove, A., Jones, H. D., & Shewry, P. R. (2007). Promoter analysis and immunolocalisation show that puroindoline genes are exclusively expressed in starchy endosperm cells of wheat grain. Plant Molecular Biology, 64, 125–136. doi:10.1007/s11103-007-9139-x.
Yamagata, H., Tamura, K., Tanaka, K., & Kasai, Z. (1986). Cell-free synthesis of rice prolamin. Plant and Cell Physiology, 27, 1419–1422.
Hood, E. E., Witcher, D. R., & Maddoock, S. (1997). Commercial production of avidin from transgenic maize: Characterization of transformant, production, processing, extraction and purification. Molecular Breeding, 3, 291–306. doi:10.1023/A:1009676322162.
Doran, P. M. (2006). Foreign protein degradation and instability in plants and plant tissue cultures. Trends in Biotechnology, 24, 426–432.
Fischer, R., & Emans, N. (2000). Molecular farming of pharmaceutical proteins. Transgenic Research, 9, 279–299. doi:10.1023/A:1008975123362.
Bowman, C. C., & Clements, J. D. (2001). Differential biological and adjuvant activities of cholera toxin and Escherichia coli heat-labile enterotoxin hybrids. Infection and Immunity, 69, 1528–1535. doi:10.1128/IAI.69.3.1528-1535.2001.
Acknowledgments
This research was supported by the International Cooperation Program of the Ministry of Science and Technology, Republic of Korea, and the Bilateral Intergovernmental Science & Technology Cooperation, KOR 13/99. This study was also supported by the Hungarian Scientific Research Fund (OTKA T 034791) and, in part, by ICGEB (CRP/HUN00-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oszvald, M., Kang, TJ., Tomoskozi, S. et al. Expression of Cholera Toxin B Subunit in Transgenic Rice Endosperm. Mol Biotechnol 40, 261–268 (2008). https://doi.org/10.1007/s12033-008-9083-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-008-9083-2