Abstract
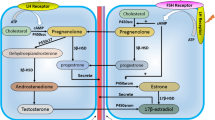
Androgens have been associated with the development of normal breast, and their role in mammary gland carcinogenesis has also been described. Several studies reported that androgens inhibit breast cancer cell growth, whereas others linked their action with the modulation of calcium (Ca2+) pumps, Ca2+ channels and Ca2+-binding proteins. Also, it is known that deregulated Ca2+ homeostasis has been implicated in the pathophysiology of breast. The L-type Ca2+ channels (LTCCs) were found to be up-regulated in colon, colorectal and prostate cancer, but their presence in breast tissues remains uncharacterized. On the other hand, regucalcin (RGN) is a Ca2+-binding protein involved in the control of mammary gland cell proliferation, which has been identified as an androgen target gene in distinct tissues except breast. This study aimed to confirm the expression and activity of LTCCs in human breast cancer cells and investigate the effect of androgens in regulating the expression of α1C subunit (Cav1.2) of LTCCs and Ca2+-binding protein RGN. PCR, Western blot, immunofluorescence and electrophysiological experiments demonstrated the expression and activity of Cav1.2 subunit in MCF-7 cells. The MCF-7 cells were treated with 1, 10 or 100 nM of 5α-dihydrotestosterone (DHT) for 24–72 h. The obtained results showed that 1 nM DHT up-regulated the expression of Cav1.2 subunit while diminishing RGN protein levels, which was underpinned by reduced cell viability. These findings first confirmed the presence of LTCCs in breast cancer cells and opened new perspectives for the development of therapeutic approaches targeting Ca2+ signaling.






Similar content being viewed by others
References
Dimitrakakis C, Bondy C. Androgens and the breast. Breast Cancer Res. 2009;11(5):212.
Greeve MA, Allan RK, Harvey JM, Bentel JM. Inhibition of MCF-7 breast cancer cell proliferation by 5alpha-dihydrotestosterone; a role for p21(Cip1/Waf1). J Mol Endocrinol. 2004;32(3):793–810.
Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, Van den Berg M. Effect of androgens on different breast cancer cells co-cultured with or without breast adipose fibroblasts. J Steroid Biochem. 2013;138:54–62.
Ortmann J, Prifti S, Bohlmann MK, Rehberger-Schneider S, Strowitzki T, Rabe T. Testosterone and 5 alpha-dihydrotestosterone inhibit in vitro growth of human breast cancer cell lines. Gynecol Endocrinol. 2002;16(2):113–20.
Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63(2):142–8.
Dick IM, Liu J, Glendenning P, Prince RL. Estrogen and androgen regulation of plasma membrane calcium pump activity in immortalized distal tubule kidney cells. Mol Cell Endocrinol. 2003;212(1–2):11–8.
Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64(22):8365–73.
Oliver VL, Anderson C, Ventura S, Haynes JM. Androgens regulate adenylate cyclase activity and intracellular calcium in stromal cells derived from human prostate. Prostate. 2010;70(11):1222–32.
Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2004;287(5):H2091–8.
Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res. 2004;36(4):197–202.
Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: targets for breast cancer. BBA-Rev Cancer. 2006;1765(2):235–55.
Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16(3):107–21.
Wang XT, Nagaba Y, Cross HS, Wrba F, Zhang L, Guggino SE. The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. Am J Pathol. 2000;157(5):1549–62.
Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997;276(5316):1268–72.
Chen R, Zeng X, Zhang R, Huang J, Kuang X, Yang J, Liu J, Tawfik O, Thrasher JB, Li B. Cav1.3 channel alpha1D protein is overexpressed and modulates androgen receptor transactivation in prostate cancers. Urol Oncol. 2014;32(5):524–36.
Taylor JM, Simpson RU. Inhibition of cancer cell-growth by calcium-channel antagonists in the athymic mouse. Cancer Res. 1992;52(9):2413–8.
Marques R, Maia CJ, Vaz C, Correia S, Socorro S. The diverse roles of calcium-binding protein regucalcin in cell biology: from tissue expression and signalling to disease. Cell Mol Life Sci. 2014;71(1):93–111.
Vaz CV, Maia CJ, Marques R, Gomes IM, Correia S, Alves MG, Cavaco JE, Oliveira PF, Socorro S. Regucalcin is an androgen-target gene in the rat prostate modulating cell-cycle and apoptotic pathways. Prostate. 2014;74(12):1189–98.
Laurentino SS, Correia S, Cavaco JE, Oliveira PF, Rato L, Sousa M, Barros A, Socorro S. Regucalcin is broadly expressed in male reproductive tissues and is a new androgen-target gene in mammalian testis. Reproduction. 2011;142(3):447–56.
Maia C, Santos C, Schmitt F, Socorro S. Regucalcin is under-expressed in human breast and prostate cancers: effect of sex steroid hormones. J Cell Biochem. 2009;107(4):667–76.
Marques R, Vaz CV, Maia CJ, Gomes M, Gama A, Alves G, Santos CR, Schmitt F, Socorro S. Histopathological and in vivo evidence of regucalcin as a protective molecule in mammary gland carcinogenesis. Exp Cell Res. 2015;330(2):325–35.
Esfahani A, Kendall CW, Bashyam B, Archer MC, Jenkins DJ. The effect of physiological concentrations of sex hormones, insulin, and glucagon on growth of breast and prostate cells supplemented with unmodified human serum. Vitro Cell Dev Biol Anim. 2010;46(10):856–62.
Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275–83.
Hofmann F, Flockerzi V, Kahl S, Wegener JW. L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev. 2014;94(1):303–26.
Li F, Wang W, Gu M, Gyoneva S, Zhang J, Huang S, Traynelis SF, Cai H, Guggino SE, Zhang X. L-type calcium channel activity in osteoblast cells is regulated by the actin cytoskeleton independent of protein trafficking. J Bone Miner Metab. 2011;29(5):515–25.
Yucel G, Altindag B, Gomez-Ospina N, Rana A, Panagiotakos G, Lara MF, Dolmetsch R, Oro AE. State-dependent signaling by Cav1.2 regulates hair follicle stem cell function. Genes Dev. 2013;27(11):1217–22.
Das R, Burke T, Van Wagoner DR, Plow EF. L-type calcium channel blockers exert an antiinflammatory effect by suppressing expression of plasminogen receptors on macrophages. Circ Res. 2009;105(2):167–75.
Taylor JT, Huang L, Pottle JE, Liu K, Yang Y, Zeng X, Keyser BM, Agrawal KC, Hansen JB, Li M. Selective blockade of T-type Ca2+ channels suppresses human breast cancer cell proliferation. Cancer Lett. 2008;267(1):116–24.
Sieber M, Nastainczyk W, Zubor V, Wernet W, Hofmann F. The 165-kDa peptide of the purified skeletal muscle dihydropyridine receptor contains the known regulatory sites of the calcium channel. Eur J Biochem. 1987;167(1):117–22.
Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127(3):591–606.
Gomez-Ospina N, Panagiotakos G, Portmann T, Pasca SP, Rabah D, Budzillo A, Kinet JP, Dolmetsch RE. A promoter in the coding region of the calcium channel gene CACNA1C generates the transcription factor CCAT. PLoS ONE. 2013;8(4):e60526.
Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330(6000):105–9.
Gry M, Rimini R, Stromberg S, Asplund A, Ponten F, Uhlen M, Nilsson P. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genom. 2009;10:365.
Ando S, De Amicis F, Rago V, Carpino A, Maggiolini M, Panno ML, Lanzino M. Breast cancer: from estrogen to androgen receptor. Mol Cell Endocrinol. 2002;193(1–2):121–8.
Capiod T. The need for calcium channels in cell proliferation. Recent Pat Anticancer Drug Discov. 2013;8(1):4–17.
Wang X, Gerard C, Theriault JF, Poirier D, Doillon CJ, Lin SX. Synergistic control of sex hormones by 17beta-HSD type 7: a novel target for estrogen-dependent breast cancer. J Mol Cell Biol. 2015;. doi:10.1093/jmcb/mjv028.
Marino AA, Iliev IG, Schwalke MA, Gonzalez E, Marler KC, Flanagan CA. Association between cell membrane potential and breast cancer. Tumour Biol. 1994;15(2):82–9.
Doering CJ, Zamponi GW. Molecular pharmacology of high voltage-activated calcium channels. J Bioenerg Biomembr. 2003;35(6):491–505.
Yang S, Huang XY. Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem. 2005;280(29):27130–7.
Dixon CJ, Bowler WB, Fleetwood P, Ginty AF, Gallagher JA, Carron JA. Extracellular nucleotides stimulate proliferation in MCF-7 breast cancer cells via P2-purinoceptors. Br J Cancer. 1997;75(1):34–9.
Ichikawa J, Kiyohara T. Suppression of EGF-induced cell proliferation by the blockade of Ca2+ mobilization and capacitative Ca2+ entry in mouse mammary epithelial cells. Cell Biochem Funct. 2001;19(3):213–9.
Chang HT, Huang JK, Wang JL, Cheng JS, Lee KC, Lo YK, Liu CP, Chou KJ, Chen WC, Su W, Law YP, Jan CR. Tamoxifen-induced increases in cytoplasmic free Ca2+ levels in human breast cancer cells. Breast Cancer Res Treat. 2002;71(2):125–31.
Vaz CV, Rodrigues DB, Socorro S, Maia CJ. Effect of extracellular calcium on regucalcin expression and cell viability in neoplastic and non-neoplastic human prostate cells. Biochim Biophys Acta. 2015;. doi:10.1016/j.bbamcr.2015.07.006.
Nakagawa T, Yamaguchi M. Overexpression of regucalcin enhances its nuclear localization and suppresses L-type Ca2+ channel and calcium-sensing receptor mRNA expressions in cloned normal rat kidney proximal tubular epithelial NRK52E cells. J Cell Biochem. 2006;99(4):1064–77.
Yamaguchi M, Kurota H. Expression of calcium-binding protein regucalcin mRNA in the kidney cortex of rats: the stimulation by calcium administration. Mol Cell Biochem. 1995;146(1):71–7.
Acknowledgments
This work was partially supported by the Portuguese Foundation for Science and Technology (FCT) under Program COMPETE (PEst-OE/SAU/UI0709/2014). Cátia V. Vaz and Ricardo Marques were funded by FCT fellowships (SFRH/BD/70316/2010 and SFRH/BD/66875/2009, respectively).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Marques, R., Peres, C.G., Vaz, C.V. et al. 5α-Dihydrotestosterone regulates the expression of L-type calcium channels and calcium-binding protein regucalcin in human breast cancer cells with suppression of cell growth. Med Oncol 32, 228 (2015). https://doi.org/10.1007/s12032-015-0676-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0676-x