Abstract
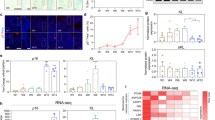
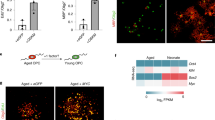
Klotho functions as an aging suppressor, which, in mice, extends lifespan when overexpressed and accelerates development of aging-like phenotypes when disrupted. Klotho is mainly expressed in brain and kidney and is secreted into the serum and CSF. We have previously shown that Klotho is reduced in brains of old monkeys, rats, and mice. We further reported the ability of Klotho to enhance oligodendrocyte differentiation and myelination. Here, we examined the signaling pathways induced by Klotho in MO3.13, a human oligodendrocytic hybrid cell line. We show that exogenous Klotho affects the ERK and Akt signaling pathways, decreases the proliferative abilities and enhances differentiation of MO3.13 cells. Furthermore, microarray analysis of Klotho-treated MO3.13 cells reveals a massive change in gene expression with 80 % of the differentially expressed genes being downregulated. Using gene set enrichment analysis, we predicted potential transcription factors involved in regulating Klotho-treated MO3.13 cells and found that these cells are highly enriched in the gene sets, that are similarly observed in cancer, cardiovascular disease, stress, aging, and hormone-related chemical and genetic perturbations. Since Klotho is downregulated in all brain tumors tested to date, enhancing Klotho has therapeutic potential for treating brain and other malignancies.








Similar content being viewed by others
Abbreviations
- PCR:
-
Polymerase chain reaction
- DMEM:
-
Dulbecco’s modified Eagle’s Medium
- PBS:
-
Phosphate buffered saline
- FBS:
-
Fetal bovine serum
- BSA:
-
Bovine serum albumin
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- MAG:
-
Myelin-associated glycoprotein
- MBP:
-
Myelin basic protein
- CNP:
-
2′,3′cyclic nucleotide-3′-phosphodiesterase
- OPC:
-
Oligodendrocytic precursor cells
References
Abraham CR, Chen C, Cuny GD, Glicksman MA, Zeldich E (2012) Small-molecule Klotho enhancers as novel treatment of neurodegeneration. Future Med Chem 4:1671–1679
Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B, Wolf I (2011) KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res 17:4254–4266
Baldi P, Long A (2001) A Bayesian framework for the analysis of microarray expression data: regularized t -test and statistical inferences of gene changes. Bioinformatics 17:509–519
Boscia F, D’Avanzo C, Pannaccione A et al (2012) Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ 19:562–572
Buntinx M, Vanderlocht J, Hellings N, Vandenabeele F, Lambrichts I, Raus J, Ameloot M, Stinissen P, Steels P (2003) Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. J Neurocytol 32:25–38
Chang B, Kim J, Jeong D et al (2012) Klotho inhibits the capacity of cell migration and invasion in cervical cancer. Oncol Rep 28:1022–1028
Chateau MT, Araiz C, Descamps S, Galas S (2010) Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging (Albany NY) 2:567–581
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR (2007) Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104:19796–19801
Chen B, Ma X, Liu S, Zhao W, Wu J (2012) Inhibition of lung cancer cells growth, motility and induction of apoptosis by Klotho, a novel secreted Wnt antagonist, in a dose-dependent manner. Cancer Biol Ther 13:1221–1228
Chen CD, Sloane JA, Li H et al (2013) The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci: Off J Soc Neurosci 33:1927–1939
de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ, Levy DE, Depinho RA, Bonni A (2008) Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev 22:449–462
de Oliveira RM (2006) Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett 580:5753–5758
Dell'Albani P, Kahn MA, Cole R, Condorelli DF, Giuffrida-Stella AM, de Vellis J (1998) Oligodendroglial survival factors, PDGF-AA and CNTF, activate similar JAK/STAT signaling pathways. J Neurosci Res 54:191–205
Doi S, Zou Y, Togao O et al (2011) Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286:8655–8665
Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR (2008) Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia 56:106–117
Gan LH, Pan J, Chen SJ, Zhong J, Wang LJ (2011) DNA methylation of ZIC1 and KLOTHO gene promoters in colorectal carcinomas and its clinicopathological significance. Zhejiang Da Xue Xue Bao Yi Xue Ban 40:309–314
German DC, Khobahy I, Pastor J, Kuro OM, Liu X (2012) Nuclear localization of Klotho in brain: an anti-aging protein. Neurobiol Aging 33(1483):e1425–e1430
Guo B, Aslam F, van Wijnen AJ et al (1997) YY1 regulates vitamin D receptor/retinoid X receptor mediated transactivation of the vitamin D responsive osteocalcin gene. Proc Natl Acad Sci U S A 94:121–126
Hartmann W, Koch A, Brune H et al (2005) Insulin-like growth factor II is involved in the proliferation control of medulloblastoma and its cerebellar precursor cells. Am J Pathol 166:1153–1162
He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave K, Casaccia-Bonnefil P (2007) The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron 55:217–230
Karin M, Yamamoto Y, Wang QM (2004) The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov 3:17–26
Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL (2009) DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 63:761–773
King GD, Chen C, Huang MM et al (2012a) Identification of novel small molecules that elevate Klotho expression. Biochem J 441:453–461
King GD, Rosene DL, Abraham CR (2012b) Promoter methylation and age-related downregulation of Klotho in rhesus monkey. Age (Dordr) 34:1405–1419
Kioussi C, Gross MK, Gruss P (1995) Pax3: a paired domain gene as a regulator in PNS myelination. Neuron 15:553–562
Kuro-o M (2009) Klotho and aging. Biochim Biophys Acta 1790:1049–1058
Kuro-o M, Matsumura Y, Aizawa H et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kurosu H, Yamamoto M, Clark JD et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833
Kurosu H, Ogawa Y, Miyoshi M et al (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123
Lee J, Jeong DJ, Kim J et al (2010) The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer 9:109
Lu L, Katsaros D, Wiley A, de la Longrais IA, Puopolo M, Yu H (2008) Klotho expression in epithelial ovarian cancer and its association with insulin-like growth factors and disease progression. Cancer Invest 26:185–192
Maekawa Y, Ishikawa K, Yasuda O et al (2009) Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 35:341–346
Mao Y, Ge X, Frank CL et al (2009) Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136:1017–1031
Massa PT, Saha S, Wu C, Jarosinski KW (2000) Expression and function of the protein tyrosine phosphatase SHP-1 in oligodendrocytes. Glia 29:376–385
Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M (2005) Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126:1274–1283
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y (1998) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242:626–630
McLaurin J, Trudel GC, Shaw IT, Antel JP, Cashman NR (1995) A human glial hybrid cell line differentially expressing genes subserving oligodendrocyte and astrocyte phenotype. J Neurobiol 26:283–293
Min C, Eddy SF, Sherr DH, Sonenshein GE (2008) NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem 104:733–744
Oh SY, Chen CD, Abraham CR (2010) Cell-type dependent modulation of Notch signaling by the amyloid precursor protein. J Neurochem 113:262–274
Pan J, Zhong J, Gan LH, Chen SJ, Jin HC, Wang X, Wang LJ (2011) Klotho, an anti-senescence related gene, is frequently inactivated through promoter hypermethylation in colorectal cancer. Tumour Biol 32:729–735
Pressinotti NC, Klocker H, Schafer G et al (2009) Differential expression of apoptotic genes PDIA3 and MAP3K5 distinguishes between low- and high-risk prostate cancer. Mol Cancer 8:130
Rubinek T, Shulman M, Israeli S et al (2012) Epigenetic silencing of the tumor suppressor klotho in human breast cancer. Breast Cancer Res Treat 133:649–657
Rummel C, Hubschle T, Gerstberger R, Roth J (2004) Nuclear translocation of the transcription factor STAT3 in the guinea pig brain during systemic or localized inflammation. J Physiol 557:671–687
Scheid MP, Marignani PA, Woodgett JR (2002) Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol 22:6247–6260
Shu G, Xie B, Ren F, Liu DC, Zhou J, Li Q, Chen J, Yuan L (2013) Restoration of klotho expression induces apoptosis and autophagy in hepatocellular carcinoma cells. Cell Oncol (Dordr) 36:121–129
Subramanian A, Tamayo P, Mootha V et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550
Torres PU, Prie D, Molina-Bletry V, Beck L, Silve C, Friedlander G (2007) Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int 71:730–737
Unger RH (2006) Klotho-induced insulin resistance: a blessing in disguise? Nat Med 12:56–57
Wang L, Wang X, Jie P et al (2011) Klotho is silenced through promoter hypermethylation in gastric cancer. Am J Cancer Res 1:111–119
Wegner M (2000) Transcriptional control in myelinating glia: the basic recipe. Glia 29:118–123
Wolf, I., Levanon-Cohen, S., Bose, S. et al. (2008) Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene
Xie B, Zhou J, Shu G, Liu DC, Chen J, Yuan L (2013) Restoration of klotho gene expression induces apoptosis and autophagy in gastric cancer cells: tumor suppressive role of klotho in gastric cancer. Cancer Cell Int 13:18
Acknowledgments
We thank Drs. Yuriy Alekseyev and Marc Lenburg at Boston University Microarray Core Facility for help with the microarray analysis. We thank Chun-Tsin Hsu for assistance with the qRT-PCR experiment and Dr. Christina Khodr for helpful discussions. This work was supported by NIH-NIA grant AG-00001 to CRA, and an Ellison Foundation Award to CC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 23 kb)
Rights and permissions
About this article
Cite this article
Chen, CD., Li, H., Liang, J. et al. The Anti-Aging and Tumor Suppressor Protein Klotho Enhances Differentiation of a Human Oligodendrocytic Hybrid Cell Line. J Mol Neurosci 55, 76–90 (2015). https://doi.org/10.1007/s12031-014-0336-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0336-1