Abstract
Myeloid derived suppressor cells (MDSCs), a heterogeneous population of myeloid progenitors, are recognized as a key element in tumor escape and progression. The importance of MDSCs in human malignancies has been demonstrated in recent years, and new approaches targeting their suppressive/tolerogenic action are currently being tested in both preclinical model and clinical trials. However, emerging evidence suggests that MDSCs may play a prominent role as regulator of the physiologic, the chronic, and the pathologic immune responses. This review will focus on the biology of MDSC in light of these new findings and the possible role of this myeloid population not only in the progression of the tumor but also in its initiation.
Similar content being viewed by others
Introduction
Myeloid derived suppressor cells (MDSCs) were initially described in the late 1970s by numerous groups [1] and described as natural suppressor cells (NS) able to inhibit the proliferative responses of T-helper lymphocytes to mitogens or alloantigens. These cells were suspected to play a key role in the induction of tolerance and in the immunosuppression induced by the tumor. Despite the importance of these early findings, many experimental limitations (i.e., a restricted antibody panel to identify their phenotype, the widespread use of culture supernatants with unknown cytokines and growth factors composition, and the absence of high purity techniques to isolate cell subsets) made confirming their very existence difficult and postponed for many years real progress in understanding their biology.
In the late 1990s, these cells were rediscovered independently by two groups [2–4]. Since then, the extraordinary importance that this cell population plays in regulating the immune system has become evident.
MDSCs phenotype
MDSCs encompass a heterogeneous population of immature and mature myeloid cells with immunoregulatory activity. This cell subset is often present in situations of immunological stress such as tumor growth [5], infections [6], or vaccination with superantigen [7], as a result of the expansion of hematopoietic precursors followed by their mobilization.
In mice, these cells can be identified by the expression of CD11b and Gr1. Co-expression of these markers, together with the immature marker CD31, and the ability to form colonies in agar is consistent with the phenotype of myeloid progenitors [2, 8, 9]. Indeed, CD11b+/Gr1+ cells in tumor-bearing hosts comprise myeloid precursors that can generate mature granulocytes, macrophages, and DCs when cultured in vitro with the appropriate cytokines cocktail [2, 10, 11].
More recently, MDSCs have been classified in two main subsets with different phenotypic and biological properties: the monocytic (mMDSC) and polymorphonuclear/granulocytic-like (gMDSC) [12–15]. In tumor-bearing mice, CD11b+Ly6ChiLy6G− mMDSCs are highly immunosuppressive and exert their effect largely in a no antigen-specific manner. By comparison, murine CD11b+Ly6CloLy6G+ gMDSC are moderately immunosuppressive and promote T cell tolerance via antigen-specific mechanisms [12–15]. The same phenotypes in tumor-free, naïve mice define, respectively, inflammatory monocytes and polymorphonuclear neutrophils both lacking the immunosuppressive activity [16]. In the vast majority of tumor models, as well as in cancer patients, gMDSC are the predominant subset [12, 17–21], representing 70–80 % of the tumor-induced MDSCs compared to 20–30 % of the cells reflecting the monocytic lineage [12, 18, 22]. However, recent evidence [23] indicates that these subsets are not two completely distinct, fully differentiated myeloid populations but rather they may represent two different differentiation states of the same population. Nevertheless, gMDSC and mMDSC have been shown to employ different mechanisms of immunosuppression (as described below), and it is important to emphasize that gMDSC and mMDSC are not inclusive of all the existing subsets.
In contrast to murine MDSCs, human MDSCs are still being phenotypically characterized because of the lack of a Gr1-like associated marker and the phenotypic variability dependent on the disease, the anatomic site, or the physiological condition of the patient. Nevertheless, a consensus is growing in defining human MDSCs as CD33+CD11b+HLA-DRlow/−. Within this population, the CD14+CD15low/− MDSCs share characteristic similar to the murine monocytic MDSCs, whereas the CD14−CD15+ MDSCs resemble the murine granulocytic subtype [24].
MDSC’s mechanisms of immunosuppression
MDSCs can restrain the immune response through different mechanisms that operate singularly or in combination. Such mechanisms can be direct (influencing directly effector T cells) or indirect. Indirect mechanisms involve the generation and/or the expansion of other regulatory populations, such as regulatory T cells.
Direct mechanisms of immunosuppression
Arginase 1 (Arg1) or liver arginase converts the semi-essential amino acid l-arginine (l-Arg) into urea and l-ornithine [25, 26]. In many different models, MDSCs can express, upon activation, high levels of this enzyme and the l-Arg transporter CAT2B. In these conditions, MDSCs readily consumed l-Arg and inhibited re-expression of the ζ-chain of CD3 complex in T lymphocytes thereby impairing their function. The CD3 ζ-chain is the main signal-transduction component of the TCR complex and is required for the correct assembly of the receptor. Interestingly, altered expression of this component has been described in peripheral blood T cells of patients with cancer, chronic infections, and autoimmune diseases [27], conditions that as described below have been associated with MDSC accumulation. In vivo, this mechanism of T cell inactivation by ARG-induced deregulation of CD3 ζ-chain seems to be relevant for tumor escape. For example, injection of the ARG inhibitor N-hydroxy-nor-l-arginine (nor-NOHA) delayed the growth of transplantable lung carcinoma in a dose-dependent manner [28]. Similarly, transgenic mice expressing high levels of Arg-1 in the enterocytes of the small intestine were shown to have serious defects in the formation of lymphoid organs and in particular of the Peyer’s patches [29]. Beside the CD3 ζ-chain down-regulation, other mechanisms seem to be involved, since T cells cultured in the absence of l-Arg had an increased production of IL-2 and expressed early activation markers [30]. Indeed, l-Arg starvation arrested T cells in the G0–G1 phase of the cell cycle, by failing to up-regulate cyclin D3 and cdk4 and increasing cdk6 expression [31]. The decreased expression of cyclin D3 and cdk4 in T cells seems to be mediated by a HUR-dependent decreased mRNA stability and diminished translational rate [32]. Moreover, under l-Arg starvation, T cells accumulate empty aminoacyl tRNAs. This accumulation activates GCN2 kinase which phosphorylates the translation initiation factor eIF2α. The phosphorylated form of eIF2α binds with high affinity to eIF2B, blocking its ability to exchange GDP for GTP, which inhibits the binding of the eIF2 complex to methionine aminoacyl tRNA resulting in a decreased initiation of global protein synthesis [30].
Nitric Oxide Synthase 2 (NOS2) oxidizes l-Arg in two steps to generate nitric oxide (NO) and citrulline [25, 26]. NOS2 is generally induced by type 1 cytokines, and it is normally associated with macrophages differentiated toward a “M1” phenotype [33]. Although NO is fundamental for its anti-microbial action and, in tumor, has been reported to have a tumoricidal action [34], its immunosuppressive role and its pro-tumoral activity are also extremely important [34]. For example, NOS inhibitors were shown to reverse MDSC-induced immunosuppression both in vivo and in vitro [26, 35]. NO seems to prevent T cell activation by interfering with the signaling cascade downstream of the IL-2 receptor (i.e., AK1, JAK3, STAT5, ERK and AKT) rather than inhibiting the early events triggered by TCR recognition [36]. NO can negatively regulate intracellular-signaling proteins either directly by S-nitrosylation of crucial cysteine residues or indirectly by activation of soluble guanylate cyclase and cyclic-GMP-dependent protein kinases [37–39]. Additionally, high concentrations of NO can exert a direct pro-apoptotic effect in T cells by mediating the accumulation of p53, by inducing FAS, or caspase-independent signaling [40, 41].
NOS2 and ARG1 have long been considered antithetic enzymes that rarely can be co-expressed in the same cells or the same microenvironment. While NOS2 is a marker of M1 macrophage, Arg1 is normally associated with a M2 phenotype [34]. However, a growing number of reports contradict this early assumption and show that these two enzymes can be co-expressed [42–50]. In these situations, ARG1, by lowering the l-Arg concentration in the local environment, operates to switch NOS2 activity, shifting its function from the production of NO to O2 − [51–53]. O2 − spontaneously reacts with other molecules (i.e., NO or H2O) and generates other reactive nitrogen intermediates (RNI), such as peroxynitrite (ONOO−), or reactive oxygen species (ROS), such as hydrogen peroxide (H2O2). These species have multiple inhibitory effects on T cells. In addition, low levels of NO induce nitrosylation of cysteine residues of ARG1, which increases the biological activity of the enzyme, further reducing l-Arg concentration in the environment [54].
Cysteine starvation Cysteine is another essential amino acid for T cell activation. Indeed, T cells lack cystathionase, the enzyme that converts methionine to cysteine and do not have an intact x −c cysteine transporter [55, 56]. Therefore, they cannot produce cysteine nor import cystine and reduce it intracellularly to cysteine. Thus, T cells depend on APCs, such as macrophages and DCs, to export cysteine, which is then imported by T cells via their ASC neutral amino acid transporter [57, 58]. MDSC play a critical role in this T cells/APCs communication, since they can drastically reduce the extracellular cysteine availability preventing T cells activation. MDSCs, in fact, do express the x −c transporter and import cystine, but they do not express the ASC transporter and, thus, cannot export it [59]. It was thus suggested that MDSCs compete with APCs for extracellular cysteine, limiting the extracellular pool of cysteine and thus depriving T cells of the cysteine they require for activation and function [59].
ROS In addition to amino acid starvation, MDSCs can block T cell function through the production of highly oxidative ROS. ROS can induce the loss of CD3 ζ chain in naive T cells [60–62]. This mechanism has been suggested in patients with pancreatic cancer in which CD11b+CD15+gMDSC were shown to reduce CD3 ζ-chain expression and decreased cytokine production in T cells through a H2O2-mediated mechanism [21]. It appears that gMDSCs have substantially higher levels of ROS and myeloperoxidase and reduced phagocytosis compared with mMDSC [63, 64]. Although the formation of ROS in myeloid cells can be mediated by the NOS2 reductase domain, NADPH oxidase (NOX) is the primary producer of ROS by catalyzing the one-electron reduction in oxygen to superoxide anion using electrons supplied by NADPH [65]. As mentioned above, one of the most common molecules that react with O2 − is NO, a key biological messenger in mammals. This leads to the formation of the free radical peroxynitrite ONOO− that can nitrate/nitrosylate tyrosine, cysteine, methionine, and tryptophan in different proteins and enzymes, thus changing their biological functions [66]. For example, peroxynitrite can nitrate/nitrosylate the TCRs and CD8 molecules on the surface of T cells. Upon this modification, the TCR loses the ability to recognize specific peptide/MHC (pMHC) complexes and CTLs are therefore rendered incompetent in performing their anti-tumor activity [67]. Alternatively, peroxynitrite can nitrate/nitrosylate chemokines within the tumor microenvironment [68]. Nitrosylated chemokines (i.e., CCL2) failed to attract T cells to the tumor, while it was still able to promote the MDSC trafficking to the tumor [68]. Finally, peroxynitrite can inhibit the binding of processed peptides to tumor cell MHC rendering the tumor invisible and resistant to antigen-specific CTLs [69].
Indirect mechanism of immunosuppression: MDSCs as tolerogenic APC
MDSCs share many features with tolerogenic DCs (e.g., antigen uptake capacity, common surface markers, cytokine profile, etc.) that have often been proposed to be associated with either T cell tolerization or Treg cell expansion. We recently performed a transcriptome and positioning analysis using RNA from in vitro differentiated human MDSCs and publically available genechip databases to define plasmacytoid and myeloid dendritic cells, monocyte-derived immature DCs, monocytes, M1 and M2 macrophage, and MDSCs from patients affected by sarcoma (Zoso et al. submitted). This analysis reveals, as expected, that, in vitro, differentiated MDSCs cluster with the tumor-derived counterpart. Although MDSCs share many clusters of genes with M1 and M2 macrophages, they are closer to the DC macro-group than to the one that includes monocytes, immature DC, and macrophages (Zoso et al. submitted). This result is not completely surprising considering our earlier report showing that MDSCs are the tolerogenic APC in lymphoma bearing mice [70]. Using the A20 B cell lymphoma model, we showed that MDSCs are capable of antigen uptake and presentation to tumor-specific Treg by a mechanism that requires ARG but is TGF-β-independent [70]. In vitro and in vivo inhibition of MDSC function, with either NOHA or Sildenafil, abrogates Treg proliferation and tumor-induced tolerance in antigen-specific T cells [70]. More recently, the expression of the immune stimulatory receptor CD40 on MDSCs was shown to be required to induce T cell tolerance and Treg accumulation [71]. While the adoptive transfer of wild-type Gr1+CD115+MDSC-induced Treg differentiation, CD40−/−MDSCs failed to induce tolerance and Treg accumulation in vivo [71]. Other reports seem to confirm the tolerogenic role of MDSCs. For example, in an allogeneic BM transplantation setting [72], CD11b+/Gr1+ MDSCs, expanded in vivo by Progenipoietin-1 (a synthetic G-CSF/Flt-3 ligand molecule) administration, were found to suppress the initiation of graft-versus-host disease (GVHD). The treatment was found to induce in the recipient a population of MHC class II-restricted, IL-10 producing Treg [72]. Similarly, we showed that induction of MDSCs via G-CSF administration is sufficient to significantly delay skin allograft rejection by a mechanism that involved the generation of regulatory T cells [73].
The importance of CD11b+cells in controlling Treg homeostasis was also shown in a melanoma mouse model and a colon carcinoma rat model [74]. In these models, Treg accumulate in the growing tumors and secondary lymphoid organs through a mechanism that mainly required the proliferation of preexisting natural Treg and the presence of CD11b+MDSC-like cells in the draining lymph nodes and in the tumor bed [74, 75]. The ability of MDSCs to induce proliferation/conversion of Tregs was recently confirmed in human setting: CD14+HLADR−/low mMDSCs isolated from PBMCs of patients with hepatocellular carcinoma were shown to induce IL-10 producing CD4+CD25+Foxp3+ Tregs when co-cultured with autologous CD3/CD28-stimulated T cells [76]. These mMDSCs expressed high levels of ARG1 that is required for their suppressive activity [76]. Interestingly, while CD14+HLA-DR−/low mMDSCs induce Foxp3+Treg, CD14+HLA-DRhigh monocytes promote the generation of Th17 cells [77]. Furthermore, MDSCs seem to modulate not only the de novo induction of Tregs from CD4+ T cells, but, also, to catalyze the trans-differentiation of Foxp3+Treg from Th17 cells through a mechanism that is dependent on MDSC-derived TGF-β and retinoic acid [77].
The putative physiological role of MDSC
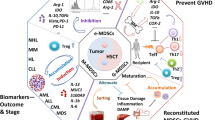
Although most of the work to describe and understand MDSCs has been performed in cancer settings, more and more data indicate that expansion of this immature myeloid cell population occurs not only in cancer, but rather their temporally defined generation, recruitment, and activation represent a normal physiological response that happens during each inflammatory response (Fig. 1a). Indeed, MDSCs may be an integral part of any immune response evolutionarily designed to prevent the excessive inflammation and the bystander damages to the tissues caused by activated T cells once the foreign antigens have been cleared. In fact, the consequences of massive T lymphocyte activation can be disastrous and can be illustrated by the severe complications seen with graft-versus-host disease in allogeneic bone marrow transplantation or in septic shock induced by bacterial toxins acting as superantigens.
Schematic representation of the role of MDSCs during a physiological immune response, a chronic inflammation, or during carcinogenesis. a Physiological immune response. Pro-inflammatory signals are released by cells infected by a pathogen or bystander cells to recruit effector T cells, immature DC, and macrophage. The immune response evolves by activating and expanding effector T cells through DC cross-presentation in the draining lymph nodes. Upon activation T cells kill the infected cells and secrete GM-CSF and other pro-inflammatory cytokines. When the pro-inflammatory cytokines reached a determinate concentration, MDSCs are recruited to turn off the immune response and promote tissue remodeling and repair. b Chronic inflammation. Upon initiation of the infection, effector cells are unable to clear the antigenic source. The high concentration of pro-inflammatory factors induced an early recruitment of MDSCs that inhibit the T cell response reaching equilibrium between pathogen, effector T cells, and MDSCs. In these situations, MDSCs actively secrete ROS and RNI that can induce mutations in the bystander cells. c Carcinogenesis. Persistent production of RNI and ROS by MDSCs in the tissue can promote mutation in oncogenes and tumor suppressor genes, thus promoting cancer initiation. Once the tumor is established, neoplastic cells produced factors able to recruit additional MDSCs that assist them in escaping from the immune recognition, in invading the surrounding tissues, and in seeding to distal site
For example, superantigen-induced activation can involve up to 20 % of the CD4+ T cells in the peripheral repertoire and cause a deadly toxic shock syndrome [78]. These immune responses are normally tightly controlled through the deletion or tolerance induction of reactive lymphocytes by a mechanism requiring an accumulation of Gr-1+ MDSC and their NO production [78]. Blocking this pathway in vivo by L-NMMA administration was shown to be sufficient to exacerbate the septic shock causing the death of most treated animals [78]. In accordance with this MDSC putative role as controller of the immune activation, we previously described the important role that GM-CSF plays in MDSCs biology. GM-CSF is a cytokine that is produced, upon activation, by virtually all the immune effector cells (i.e., T and B cells, NK cells, B cells, and DC) [79]. At low doses, it is responsible for the recruitment and stimulation of APC and to enhance the immune response. Indeed, for a long time, GM-CSF has been thought to be an extremely important immune adjuvant. However, we demonstrated that MDSCs are recruited at the vaccination site and can inhibit the immune response when GM-CSF concentration reached a determinate threshold [80]. In particular, utilizing a bystander vaccine strategy in which the antigen dose and steric hindrance could be maintained constant while altering the GM-CSF dose, we assessed the impact of high versus low concentrations of GM-CSF administered in a vaccine formulation on priming of anti-tumor immunity. We confirmed the efficacy of low doses GM-CSF secreting vaccine and defined a threshold above which the vaccine not only lost its efficacy but also resulted in significant in vivo immunosuppression mediated by MDSC recruitment [80]. A systematic analysis of different clinical trials performed with this cytokine suggests that the same phenomenon can take place in humans [81].
It is important to underline that the appearance of MDSCs following vaccination is not a sole property of GM-CSF-based vaccines but seems to be related to all immunological insults. For example, inoculation of a recombinant vaccinia virus (rVV) expressing the mouse IL-2 gene caused enhanced activation and expansion of cytotoxic T cells (CTL), as assessed by the marked increase in the ex vivo cytotoxic responses to vaccinia determinants and to the heterologous antigen carried by the rVV, the β-galactosidase (β-gal) from Escherichia coli [82]. Although present in the spleen in large numbers, CD8+ T cells specific for β-gal could not be re-stimulated in vitro or in vivo. Instead, stimulation with a β-gal epitope triggered their activation-induced cell death. The induction of such immune unresponsiveness was found to depend on MDSC activity [83] and to correlate with GM-CSF overproduction upon rVV vaccination. The addition of anti-GM-CSF antibodies during the vaccination phase or the depletion of MDSC prior to re-stimulation restored the CTL responses [2]. Beside vaccine settings, MDSCs are found expanded in nearly all inflammatory conditions suggesting that MDSCs may be more of a normal component of the inflammatory response [84]. For example, polymicrobial sepsis causes myeloid cell expansion in the bone marrow, spleen, and lymph nodes [85], and the same MDSC expansion was seen after burn [86] or traumatic injury [87]. Indeed, as mentioned above for the vaccination with superantigen, septic conditions can lead to an exaggerated and potentially lethal inflammatory response, blocking the MDSC expansion may also worsen outcome by promoting the inflammatory component. Indeed, it was shown that mice lacking gp130 and unable to signal through IL-6 failed to expand their MDSC population and had markedly higher mortalities to sepsis associated with increased inflammatory cytokine production [88].
In summary, MDSCs can be a normal component of an inflammatory response that upon sensing GM-CSF or other cytokines produced by activated T cells are recruited to the inflammation site to reduce the risks of collateral damages to the tissue or a lethal cytokine-induced shock.
MDSC and chronic inflammation
Chronic inflammations are an ongoing battle between a non-ending antigenic source or stimuli and the immune response and are characterized by the sustained recruitment of immune cells. Chronic inflammation can be maintained either by a chronic infection from a viral or bacterial pathogen, by the instauration of an autoimmune disease, or by the repetitive insults to the same immunogenic substance to the same site (i.e., smoking habits or pollution in the airways). In these situations, the antigenic source is not eliminated by the immune system.
Based on the putative physiological role of MDSCs, an expansion of this regulatory population is expected in all these conditions (Fig. 1b). Recent evidences suggest that this is the case and that virus and other pathogens indeed evolve to maximize the recruitment of MDSCs. Chronic infections usually promote high levels of the pro-inflammatory cytokine TNF-α and IL-1β that have been involved in MDSCs recruitment and survival [18, 89–91]. Additionally, viral products seem to have evolved to promote MDSCs recruitment and activation. For example, the core protein of hepatitis C virus (HCV), a pathogen that establish a chronic infection in 80 % of infected individuals, was shown to promote MDSCs accumulation through STAT3-dependent mechanism [92]. These MDSCs were found to be elevated in infected patients and were able to suppress T cells response by a ROS secretion [92]. Interestingly, during anti-viral therapy, MDSCs were shown to decrease in HCV patients [93]. Similar results were reported in patients infected by the human immunodeficiency virus (HIV) [6, 94]. Also in this case, anti-viral therapy was shown to drastically reduce MDSCs concentration in patients’ blood [94]. Besides producing pro-inflammatory cytokines, HIV seems to promote directly MDSC differentiation through TAT. Indeed, TAT added to healthy donors PBMCs induces the differentiation of CD33+CD11b+HLA-DR−/lowMDSCs [94].
It is important to underline that in addition to the fact that virus can express MDSCs facilitator genes, the immune response can also induce and can promote MDSCs accumulation. For example, the anti-viral immune response, rather than the virus, seems to mediate MDSC expansion in mice infected with vesicular stomatitis virus (VSV). MDSCs expansion was detected only during a prolonged infection of 5 days, while they were decreased when the infection was limited to 1 day, suggesting that MDSCs are recruited only during temporally sustained immune responses [95] or strong immunogenic reaction such as in the case of infection with vaccinia virus [96].
The link between chronic inflammation and MDSC recruitment is not limited to viral infections, but it might represent the unsuccessful attempt of the organism to halt a persistent inflammation as is suggested by the accumulation of MDSCs in a growing number of inflammatory conditions including uveoretinitis [97], Lichen Planus [98], autoimmune hepatitis [99], multiple sclerosis [100], and inflammatory bowel disease [101], as well as other chronic inflammation such rheumatoid arthritis [102] or smoking habits [103].
MDSCs and cancer
It is now generally accepted that chronic inflammation plays a key role in tumorigenesis [104]. An inflammatory microenvironment seems to be an essential component of all tumors, including some in which a direct causal relationship with inflammation is not yet proven [105].
Considering the new data mentioned above implicating an MDSC expansion and activation during chronic inflammation and considering the high production of ROS and RNI that characterized activated MDSCs, this suppressive population might be the link between inflammation and cancer (Fig. 1c). Studies using models of chronic inflammation seem to support this hypothesis demonstrating that prolonged exposure to ROS and RNI in the gastrointestinal-tract-induced DNA mutations and colon cancer in mice fed with dextran sulfate [106]. This possibility seems to be further suggested by human studies. For example, periodontal disease is significantly associated with an increased risk of lung, kidney, pancreatic, and haematological cancers [107]. Lichen Planus, that is associated with an increase MDSC concentration [98], also plays an important role in the etiology of oral squamous cell carcinoma [108]. Additionally, it is generally acknowledged that chronic inflammation plays a central role in chronic obstructive pulmonary disease (COPD), a condition associated with chronic tobacco smokers. A marked increase in MDSCs infiltrating the lungs and in circulation has been reported in smokers and COPD patients [103, 109–111]. The fact that numerous epidemiological studies have consistently linked the presence of COPD to the development of lung cancer, independently of cigarette smoking dosage [112], might support the hypothesis that chronic inflammation of the lung (caused by tobacco smoke or other agents) might increase the local concentration and number of activated MDSCs, their production of ROS and RNI (found in COPD patients [113, 114]), DNA mutation and eventually cancer. However, despite this circumstantial data, the role of MDSCs as initiator of tumorigenesis still needs to be proven.
Although the role of MDSCs in cancer initiation remains to be to be confirmed, a large number of reports demonstrate that MDSCs play a key role in tumor progression and metastasis. Indeed, virtually all transplantable murine models induce MDSCs whose presence has been linked to both tumor-induced immunosuppression and metastases (reviewed in [79, 115–119]). MDSCs have been linked to tumor progression also in chemically induced cancers [120, 121] and in transgenic mice that spontaneously develop tumors [122–124]. Inhibition or depletion of MDSCs is generally associated with a reversal of tumor-induced immunosuppression, a synergy with active immunotherapy and a decrease in the metastatic disease [79, 115–119].
Recent data clearly indicate that the pro-tumoral role of MDSCs is not limited to generating a suppressive niche around the tumor, but, rather, these cells also play an important role in tumor progression and metastases even through immune-independent mechanisms. Indeed, MDSCs and tumor-associated macrophages (TAMs) seem to be the main players in the metastatic process: not only they are the most abundant innate immune cells present in several types of mouse and human cancer [125–127], but also their presence correlates with increased vascular density and worse clinical outcomes in several types of human cancer [128, 129]. For example, MDSCs and TAMs, activated through alpha chain of the IL4 receptor (IL4Rα, CD124) and CSF-1R (CD115), have been identified as essential regulators of pulmonary metastasis in mouse models of mammary carcinogenesis [130, 131]. In accordance with these results, IL4Rα inactivation by pharmacologic or genetic means is sufficient to promote tumor immunity and restore the efficacy of immunotherapy [43]. Furthermore, CD124 signaling is essential for MDSCs and TAM survival as we demonstrated by the in vitro and in vivo use of an IL4Rα-specific blocking aptamer [132]. Chronic administration of anti-IL4Rα aptamer induces apoptosis in MDSCs and TAMs and reduces primary tumor growth and the number of metastatic cells in the lung of mice bearing a mammary carcinoma [132].
The pro-metastatic activity of MDSCs and TAMs is also linked to their tissue-remodeling properties. Upon activation, these leukocytes secrete matrix remodeling proteases and serine proteases that are associated with more advanced tumor grades and metastasis [133–135]. Additionally, following IL4Rα engagement, TAMs and MDSCs express elevated levels of the cysteine protease cathepsin B and expression of this protease is found within macrophages at the invasive edge of pancreatic cancers [133–135]. Metalloproteinase (MMP) and cathepsin B secretion by TAMs and MDSCs are partially regulated by IL-6 [136]. It is important to note that this cytokine, in concert with GM-CSF, is one of the key elements that regulate MDSC differentiation [137]. In particular, GM-CSF, G-CSF, and IL-6 allowed a rapid generation of MDSCs from precursors present in mouse and human bone marrow (BM) [137].
Several other studies also suggest a role of myeloid cell subsets in either promoting the formation of a pre-metastatic niche before the neoplastic cells seed at the distal site or in favoring tumor growth once the metastatic cells have been seeded [117]. According to these studies, the primary tumor appears to “prepare” the distal site for the growth of the metastasis. For example, CD11b+Gr1+MDSCs have been shown to activate the pre-metastatic lung into a permissive haven by diminishing immune-protective programs [138]. In agreement with this hypothesis, data from our laboratory indicate that MDSCs (CD11b+Gr1+IL4Rα+) represent up to 40 % of stromal cell in the lung of mice bearing the 4T1 mammary carcinoma, although only 0.1–0.5 % of neoplastic cells are present (data not shown). The recruitment of MDSCs in the pre-metastatic condition is dependent on CCL2 at least in a mammary carcinoma model [139]. Inhibition of the CCR-2/CCL2 signaling in fact drastically reduced the recruitment of Gr1+ myeloid cells to the lung and, more importantly, the number and size of metastasis [139].
MDSCs and macrophages, not only may prepare the secondary site for the seeding of the metastatic cells but also can promote the survival and the growth of seeded neoplastic cells by different mechanisms: (1) provide a localized immune-suppression that protects the secondary disease from immune clearance; (2) promote secondary tumor angiogenesis by regulating VEGF bioavailability through the secretion of MMP9 and by being incorporated in the tumor vessels (although this remains a controversial issue); (3) facilitate the invasion in the surrounding tissue promoting its remodeling [117]. Finally, (4) specific genetic ablation in a mouse model of mammary carcinoma demonstrated that a peculiar population of CD11b+ macrophages also inhibits the spontaneous apoptosis of metastatic cells in the lung [140]. These data seem to be confirmed by previous studies in which IL-1β secreted by “tumor educated” macrophages [141] has an anti-apoptotic effect on the neoplastic cells by promoting Wnt signaling in colon carcinoma.
Despite the plasticity of MDSCs and the variation in their phenotype in different human malignancies, in the recent years much progress has been made in understanding their role in human cancer. For example, in a seminal work in head and neck cancer patients, the release GM-CSF and the tumor infiltration with CD34+MDSC were determined to be negative prognostic factors because both events were associated with an increased rate of tumor and metastasis recurrence [142]. A more extensive study identified human MDSCs in the peripheral blood of patients with squamous cell carcinoma, head and neck cancer, breast cancer, and non-small-cell lung cancer [4]. Analysis of PBMCs, from patients affected by metastatic adenocarcinomas of the pancreas, colon, and breast, revealed an increase in the oxidative activity of CD15+granulocytes that resulted in an elevated ROS production. Granulocyte activation correlated with the inhibition of CD3 ζ-chain expression and cytokine production [21].
The evaluation of MDSCs in patients with different solid tumors (mostly breast and gastrointestinal tumors, but also including melanomas and other cancers), clinical stages I–IV [20], demonstrates that MDSC levels were significantly higher in cancer patients relative to healthy controls (p < 0.0001) and that their concentration was proportional to clinical cancer stage. Similar data were reported in glioblastoma, breast, colon, lung, and kidney cancer (reviewed in [5]). In breast and colorectal cancer patients, MDSC levels are indicative of the overall tumor burden and their increased circulating levels correlates with worse prognosis and radiographic progression [143, 144]. In a large study performed in metastatic renal cell carcinoma, Zea et al. [145] evaluated PBMCs from 123 patients and detected an increase in ARG activity that was associated with the down-regulation of the CD3 ζ-chain expression and reduction in IL-2 and IFN-γ production by anti-CD3/anti-CD28 stimulated PBMCs [145]. Cell fractionation studies revealed that ARG activity was limited to CD11b+CD15+CD14− gMDSCs and depletion of CD11b+cell from PBMCs was sufficient to restore ζ-chain expression, cytokine production and proliferation of otherwise anergic T cells present among PBMCs [145]. In a large study on hepatocellular carcinoma patients, increased levels were found of ARG-expressing m-MDSCs (CD14+HLADR−/low), capable of suppressing T cell proliferation [146]. In multiple myeloma and HNSCC, depletion or pharmacological inhibition of mMDSC was sufficient to restore the otherwise anergic phenotype of PBMCs [49]. Similar findings were shown in a clinical trial in which stage IV melanoma patients were vaccinated with the heat-shock protein gp96, with or without GM-CSF as adjuvant to better prime the immune response [81]. Recently, phase I/II clinical trials showed that vaccines based on tumor-associated peptides could prolong survival in patients with renal cell cancer and colorectal cancer that showed signs of a multipeptide-specific immunization [147, 148]. Moreover, positive and negative predictors of clinical responses could be found in the blood among leukocyte subsets (Treg and MDSCs) and serum proteins (chemokines and apolipoproteins) [147, 148]. In this study, a panel of antibodies was developed to identify six MDSC phenotypes in a single multicolor staining. Levels of all MDSC subsets, except one, were significantly increased in the blood of patients with renal cell cancer, suggesting a global modification of myelopoiesis in these patients. However, in a retrospective analysis, only two MDSC phenotypes were significantly negatively associated with survival: CD14+HLA-DR−/low and CD11b+CD14−CD15+ [147]. Taken together, the existing data on human MDSCs indicate that these cells share many of the functional properties found in mice. However, it is still very problematic to associate a unique panel of markers to human MDSCs. This difficulty can depend on the great plasticity and accepted heterogeneity that characterize MDSCs.
Conclusions and current directions
MDSCs are being recognized as important players in the fine mechanisms that regulate the immune response in physiological situations as well as in different pathologies. Indeed, it is now clear that blocking and inactivating an ongoing immune response is as complex as its initiation. Multiple cellular players, cytokines and chemokines, and intra- and inter-cellular signaling are involved. MDSCs seem to play a key role in this network and incredible progresses in understanding their biology has been made in the last 15 years. Nevertheless, the intrinsic plasticity of MDSC might be a blessing for the therapist but a curse for the experimentalist that wants to understand their biology, since a few modifications in the microenvironment can dramatically change their phenotype and function and even promote their maturation toward inflammatory anti-tumoral APCs (i.e., DC and macrophage) [149]. Because of this plasticity caution is still needed in the interpretation of in vitro data since the cytokine and chemical composition of the FCS used in the media is often unknown. Even the interaction of these cells with their plastic containers can change their phenotype. In vivo data are still needed to confirm any in vitro generated hypothesis, and cell-specific expression or knockdown of the desired genes in vivo is highly desirable to better understand MDSC biology in physiological or pathological settings. Since genetic knockout or transgenic mice require significant economic and time resources, and since this technology cannot be easily translated into the clinic, in recent years we developed different nano-tools that allow us to target specifically MDSCs in vivo. In particular, based on our experience with functionalized PAMAM dendrimers [150], we have developed a new nanoparticle that allows the specific targeting of MDSCs in vivo to either silence or up-regulate a determinant gene (Vella et al. in preparation). Additionally, we have selected aptamers that recognize specifically either the tumor-associated MDSC or both splenic and tumor-infiltrating MDSCs (Delafuente et al. in preparation). The MDSC-specific or the tumor-infiltrating MDSC-specific aptamers are currently being tested as carriers for shRNA or drugs.
Based on our preliminary data and work performed in other laboratories, we are confident that in the near future MDSC biology will be further elucidated. We foresee that powerful new therapies based on MDSC modulation might become available to resolve different pathologies including autoimmunity, chronic inflammation, and cancer.
References
Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–37.
Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96(12):3838–46.
Young MR, Kolesiak K, Wright MA, Gabrilovich DI. Chemoattraction of femoral CD34+ progenitor cells by tumor-derived vascular endothelial cell growth factor. Clin Exp Metastasis. 1999;17(10):881–8.
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–89.
Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35(2):107–15. doi:10.1097/CJI.0b013e318242169f.
Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, et al. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS. 2012;26(12):F31–7. doi:10.1097/QAD.0b013e328354b43f.
Fast DJ, Shannon BJ, Herriott MJ, Kennedy MJ, Rummage JA, Leu RW. Staphylococcal exotoxins stimulate nitric oxide-dependent murine macrophage tumoricidal activity. Infect Immun. 1991;59(9):2987–93.
Fu YX, Watson G, Jimenez JJ, Wang Y, Lopez DM. Expansion of immunoregulatory macrophages by granulocyte–macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res. 1990;50:227–34.
Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102(6):2138–45.
Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74(2):186–96.
Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, Segal DM, et al. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J Immunol. 2000;165(12):6723–30.
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802.
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell—suppressive activity. Blood. 2008;111(8):4233–44. doi:10.1182/blood-2007-07-099226.
Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35. doi:10.1002/eji.200939903.
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22(2):238–44. doi:10.1016/j.coi.2010.01.021.
Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi:10.1146/annurev.immunol.021908.132557.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi:10.1038/nri2506.
Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, et al. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40(12):3347–57. doi:10.1002/eji.201041037.
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14(24):8270–8. doi:10.1158/1078-0432.CCR-08-0165.
Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–60. doi:10.1158/0008-5472.CAN-08-1921.
Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61(12):4756–60.
Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182(9):5693–701. doi:10.4049/jimmunol.0900092.
Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013. doi:10.1038/ni.2526.
Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, et al. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130(5):1109–19. doi:10.1002/ijc.26123.
Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17.
Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–16.
Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4(9):675–87.
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–49.
de Jonge WJ, Hallemeesch MM, Kwikkers KL, Ruijter JM, de Gier-de Vries C, van Roon MA, et al. Overexpression of arginase I in enterocytes of transgenic mice elicits a selective arginine deficiency and affects skin, muscle, and lymphoid development. Am J Clin Nutr. 2002;76(1):128–40.
Raber P, Ochoa AC, Rodriguez PC. Metabolism of l-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. 2012;41(6–7):614–34. doi:10.3109/08820139.2012.680634.
Rodriguez PC, Quiceno DG, Ochoa AC. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–73. doi:10.1182/blood-2006-06-031856.
Rodriguez PC, Hernandez CP, Morrow K, Sierra R, Zabaleta J, Wyczechowska DD, et al. l-arginine deprivation regulates cyclin D3 mRNA stability in human T cells by controlling HuR expression. J Immunol. 2010;185(9):5198–204. doi:10.4049/jimmunol.1001224.
Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–61. doi:10.1038/nri3088.
Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6(7):521–34. doi:10.1038/nrc1910.
Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–54. doi:10.1038/nri1668.
Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168(2):689–95.
Fischer TA, Palmetshofer A, Gambaryan S, Butt E, Jassoy C, Walter U, et al. Activation of cGMP-dependent protein kinase Iβ inhibits interleukin 2 release and proliferation of T cell receptor-stimulated human peripheral T cells. J Biol Chem. 2001;276(8):5967–74.
Duhe RJ, Evans GA, Erwin RA, Kirken RA, Cox GW, Farrar WL. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc Natl Acad Sci USA. 1998;95(1):126–31.
Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160(12):5729–34.
Macphail SE, Gibney CA, Brooks BM, Booth CG, Flanagan BF, Coleman JW. Nitric oxide regulation of human peripheral blood mononuclear cells: critical time dependence and selectivity for cytokine versus chemokine expression. J Immunol. 2003;171(9):4809–15.
Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, et al. Fas-induced caspase denitrosylation. Science. 1999;284(5414):651–4.
Currie GA, Gyure L, Cifuentes L. Microenvironmental arginine depletion by macrophages in vivo. Br J Cancer. 1979;39(6):613–20.
Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116(10):2777–90. doi:10.1172/JCI28828.
Brys L, Beschin A, Raes G, Ghassabeh GH, Noel W, Brandt J, et al. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol. 2005;174(10):6095–104.
Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201(8):1257–68. doi:10.1084/jem.20042028.
Bruch-Gerharz D, Schnorr O, Suschek C, Beck KF, Pfeilschifter J, Ruzicka T, et al. Arginase 1 overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. Am J Pathol. 2003;162(1):203–11.
Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. l-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24(6):302–6.
De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci USA. 2005;102(11):4185–90. doi:10.1073/pnas.0409783102.
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–702. doi:10.1084/jem.20061104.
Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174(8):4880–91.
Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273(35):22635–9.
Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA. 1997;94(13):6954–8.
Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170(1):270–8.
Santhanam L, Lim HK, Miriel V, Brown T, Patel M, Balanson S, et al. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101(7):692–702. doi:10.1161/CIRCRESAHA.107.157727.
Gmunder H, Eck HP, Droge W. Low membrane transport activity for cystine in resting and mitogenically stimulated human lymphocyte preparations and human T cell clones. Eur J Biochem. 1991;201(1):113–7.
Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984;779(3):289–306.
Iwata S, Hori T, Sato N, Ueda-Taniguchi Y, Yamabe T, Nakamura H, et al. Thiol-mediated redox regulation of lymphocyte proliferation. Possible involvement of adult T cell leukemia-derived factor and glutathione in transferrin receptor expression. J Immunol. 1994;152(12):5633–42.
Gmunder H, Eck HP, Benninghoff B, Roth S, Droge W. Macrophages regulate intracellular glutathione levels of lymphocytes. Evidence for an immunoregulatory role of cysteine. Cell Immunol. 1990;129(1):32–46.
Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi:10.1158/0008-5472.CAN-09-2587.
Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci USA. 1996;93(23):13119–24.
Kono K, Ressing ME, Brandt RM, Melief CJ, Potkul RK, Andersson B, et al. Decreased expression of signal-transducing zeta chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin Cancer Res. 1996;2(11):1825–8.
Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26(6):1308–13.
Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91(1):167–81. doi:10.1189/jlb.0311177.
Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89(2):311–7. doi:10.1189/jlb.0310162.
Lu T, Gabrilovich DI. Molecular pathways: tumor-infiltrating myeloid cells and reactive oxygen species in regulation of tumor microenvironment. Clin Cancer Res. 2012;18(18):4877–82. doi:10.1158/1078-0432.CCR-11-2939.
Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25(3–4):295–311. doi:10.1007/s00726-003-0018-8.
Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8(+) T cell tolerance in cancer. Nat Med. 2007;13(7):828–35.
Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208(10):1949–62. doi:10.1084/jem.20101956.
Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121(10):4015–29. doi:10.1172/JCI45862.
Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–49. doi:10.1158/0008-5472.CAN-07-6621.
Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70(1):99–108. doi:10.1158/0008-5472.CAN-09-1882.
MacDonald KP, Rowe V, Clouston AD, Welply JK, Kuns RD, Ferrara JL, et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174(4):1841–50.
Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L. In vivo induction of myeloid suppressor cells and CD4(+) Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 2011;20(6):941–54. doi:10.3727/096368910X540621.
Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, et al. Tumor cells convert immature myeloid dendritic cells into TGF-β—secreting cells inducing CD4+ CD25+ regulatory T cell proliferation. J Exp Med. 2005;202(7):919–29.
Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+ CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–31. doi:10.1158/0008-5472.CAN-05-1299.
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+) CD25(+) Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–43. doi:10.1053/j.gastro.2008.03.020.
Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117(24):6532–41. doi:10.1182/blood-2010-11-317321.
Cauley LS, Miller EE, Yen M, Swain SL. Superantigen-induced CD4 T cell tolerance mediated by myeloid cells and IFN-γ. J Immunol. 2000;165(11):6056–66.
Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16(1):53–65. doi:10.1016/j.semcancer.2005.07.005.
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–43. doi:10.1158/0008-5472.CAN-04-0757.
Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18(2):226–32.
Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, et al. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154(10):5282–92.
Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161(10):5313–20.
Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17(3–4):281–92. doi:10.2119/molmed.2010.00178.
Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, et al. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184(5):2247–51. doi:10.4049/jimmunol.0903652.
Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21(5):415–25.
Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176(4):2085–94.
Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207(7):1453–64. doi:10.1084/jem.20091474.
Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14(5):408–19. doi:10.1016/j.ccr.2008.10.011.
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Investig. 2012;122(11):4094–104. doi:10.1172/JCI64115.
Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176(1):284–90.
Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, et al. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55(2):343–53. doi:10.1002/hep.24700.
Cai W, Qin A, Guo P, Yan D, Hu F, Yang Q, et al. Clinical significance and functional studies of myeloid-derived suppressor cells in chronic hepatitis C patients. J Clin Immunol. 2013;33(4):798–808. doi:10.1007/s10875-012-9861-2.
Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. 2013;87(3):1477–90. doi:10.1128/JVI.01759-12.
Liu C, Zhang C, Lu H, Cai J, Wang Z, Chen J, et al. Poly(I:C) induce bone marrow precursor cells into myeloid-derived suppressor cells. Mol Cell Biochem. 2011;358(1–2):317–23. doi:10.1007/s11010-011-0982-3.
Fortin C, Huang X, Yang Y. NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells. J Immunol. 2012;189(4):1843–9. doi:10.4049/jimmunol.1200584.
Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31(4):354–61. doi:10.1016/j.jaut.2008.08.006.
Vered M, Furth E, Shalev Y, Dayan D. Inflammatory cells of immunosuppressive phenotypes in oral lichen planus have a proinflammatory pattern of expression and are associated with clinical parameters. Clin Oral Investig. 2013;17(5):1365–73. doi:10.1007/s00784-012-0814-1.
Cripps JG, Wang J, Maria A, Blumenthal I, Gorham JD. Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology. 2010;52(4):1350–9. doi:10.1002/hep.23841.
Bowen JL, Olson JK. Innate immune CD11b+ Gr-1+ cells, suppressor cells, affect the immune response during Theiler’s virus-induced demyelinating disease. J Immunol. 2009;183(11):6971–80. doi:10.4049/jimmunol.0902193.
Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135(3):871–81, 81 e1–e5. doi:10.1053/j.gastro.2008.06.032.
Jiao Z, Hua S, Wang W, Wang H, Gao J, Wang X. Increased circulating myeloid-derived suppressor cells correlated negatively with Th17 cells in patients with rheumatoid arthritis. Scand J Rheumatol. 2013;42(2):85–90. doi:10.3109/03009742.2012.716450.
Scrimini S, Pons J, Agusti A, Soriano JB, Cosio BG, Torrecilla JA et al. Differential effects of smoking and COPD upon circulating myeloid derived suppressor cells. Respiratory medicine. 2013. doi:10.1016/j.rmed.2013.08.002.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi:10.1016/j.cell.2010.01.025.
Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22(1):33–40. doi:10.1016/j.semcancer.2011.12.005.
Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Investig. 2008;118(7):2516–25. doi:10.1172/JCI35073.
Migliorati CA. Periodontal diseases and cancer. Lancet Oncol. 2008;9(6):510–2. doi:10.1016/S1470-2045(08)70138-4.
Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral Oncol. 2013;49(9):887–92. doi:10.1016/j.oraloncology.2013.07.003.
Cosio MG, Saetta M. Evasion of COPD in smokers: at what price? Eur Respir J. 2012;39(6):1298–303. doi:10.1183/09031936.00135711.
Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–82. doi:10.1016/j.cell.2010.02.029.
Balamayooran G, Batra S, Cai S, Mei J, Worthen GS, Penn AL, et al. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am J Respir Cell Mol Biol. 2012;47(1):104–11. doi:10.1165/rcmb.2011-0260OC.
Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13(4):233–45. doi:10.1038/nrc3477.
Loukides S, Bakakos P, Kostikas K. Oxidative stress in patients with COPD. Curr Drug Targets. 2011;12(4):469–77.
Kostikas K, Papatheodorou G, Psathakis K, Panagou P, Loukides S. Oxidative stress in expired breath condensate of patients with COPD. Chest. 2003;124(4):1373–80.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi:10.1038/nri3175.
Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68(8):2561–3. doi:10.1158/0008-5472.CAN-07-6229.
Chioda M, Peranzoni E, Desantis G, Papalini F, Falisi E, Solito S, et al. Myeloid cell diversification and complexity: an old concept with new turns in oncology. Cancer Metastasis Rev. 2011;30(1):27–43. doi:10.1007/s10555-011-9268-1.
Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother. 2001;24(6):431–46.
Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79.
Horiguchi S, Petersson M, Nakazawa T, Kanda M, Zea AH, Ochoa AC, et al. Primary chemically induced tumors induce profound immunosuppression concomitant with apoptosis and alterations in signal transduction in T cells and NK cells. Cancer Res. 1999;59(12):2950–6.
Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol. 2007;7(2):140–51.
Liu Y, Van Ginderachter JA, Brys L, De Baetselier P, Raes G, Geldhof AB. Nitric oxide-independent CTL suppression during tumor progression: association with arginase-producing (M2) myeloid cells. J Immunol. 2003;170(10):5064–74.
Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189(12):5602–11. doi:10.4049/jimmunol.1201018.
Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol. 2012;22(4):319–26. doi:10.1016/j.semcancer.2012.02.003.
Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Investig. 2007;117(5):1155–66. doi:10.1172/JCI31422.
Erez N, Coussens LM. Leukocytes as paracrine regulators of metastasis and determinants of organ-specific colonization. Int J Cancer. 2011;128(11):2536–44. doi:10.1002/ijc.26032.
Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. doi:10.1038/nrc1256.
Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–85. doi:10.1056/NEJMoa0905680.
Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21(1):41–8. doi:10.1016/j.cytogfr.2009.11.009.
Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193(6):727–40.
DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi:10.1016/j.ccr.2009.06.018.
Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Research online first. 2012. doi:10.1158/0008-5472.can-11-2772.
Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–90.
Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10(4):329–40.
Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Rep. 2010;23(3):615–9.
Mohamed MM, Cavallo-Medved D, Rudy D, Anbalagan A, Moin K, Sloane BF. Interleukin-6 increases expression and secretion of cathepsin B by breast tumor-associated monocytes. Cell Physiol Biochem. 2010;25(2–3):315–24. doi:10.1159/000276564.
Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity. 2010;32(6):790–802. doi:10.1016/j.immuni.2010.05.010.
Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+ CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70(15):6139–49. doi:10.1158/0008-5472.CAN-10-0706.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. doi:10.1038/nature10138.
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE. 2009;4(8):e6562. doi:10.1371/journal.pone.0006562.
Kaler P, Galea V, Augenlicht L, Klampfer L. Tumor associated macrophages protect colon cancer cells from TRAIL-induced apoptosis through IL-1β-dependent stabilization of Snail in tumor cells. PLoS ONE. 2010;5(7):e11700. doi:10.1371/journal.pone.0011700.
Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74(1):69–74.
Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118(8):2254–65. doi:10.1182/blood-2010-12-325753.
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi:10.1007/s00262-008-0523-4.
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044–8. doi:10.1158/0008-5472.CAN-04-4505.
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50(3):799–807. doi:10.1002/hep.23054.
Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012. doi:10.1038/nm.2883.
Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Reports. 2012;2(3):628–39. doi:10.1016/j.celrep.2012.08.006.
Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–75. doi:10.1002/eji.201040895.
Daftarian P, Kaifer AE, Li W, Blomberg BB, Frasca D, Roth F, et al. Peptide-conjugated PAMAM dendrimer as a universal DNA vaccine platform to target antigen-presenting cells. Cancer Res. 2011;71(24):7452–62. doi:10.1158/0008-5472.CAN-11-1766.
Acknowledgments
This work is supported by the Flight Attendant Medical Research institute, by the DOD-BCRP-idea award and by the Bankhead Coley Cancer Research Program. The author would like to thank Donald T. Weed and Alessia Zoso for the critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serafini, P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol Res 57, 172–184 (2013). https://doi.org/10.1007/s12026-013-8455-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-013-8455-2