Abstract
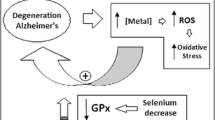
The aim of the study was to evaluate blood selenium and antioxidants as possible oxidative stress markers in Alzheimer’s disease (AD) along with amyloid β42 (Aβ42) and tau by comparing them with vascular dementia (VD) and age-matched healthy controls. Selenium, total tau, Aβ42, glutathione (GSH) and malondialdehyde (MDA) levels and the activities of antioxidant enzymes were analysed in the blood of AD patients (n = 30), VD patients (n = 35) and controls (n = 40) from South India. Plasma Aβ42 level was significantly higher (P < 0.001) in both AD and VD compared to controls. Total tau and tau-to-amyloid ratio were significantly lower in both AD and VD (P < 0.001), compared to controls, and a significant difference (P < 0.01 and P < 0.05, respectively) was also observed between AD and VD. The receiver operating characteristic (ROC) curve-derived cutoff values of <3.5 for tau-to-Aβ42 ratio and <520 pg/ml for total tau showed sensitivity and specificity of around 67–72 % for differentiating AD from VD and around 90 % for AD from controls, indicating that they could serve as reliable AD-specific markers. The MDA levels were significantly higher (P < 0.001) in both dementia groups along with a significant decrease (P < 0.001) in reduced GSH levels, indicating elevated oxidative stress and altered redox status in both forms of dementia. Selenium levels did not vary significantly between the three groups. The activity of glutathione peroxidase increased in both AD and VD compared to controls, with a concomitant decrease in glutathione reductase and glucose-6-phospate dehydrogenase (P < 0.001) activity. The activity of thioredoxin reductase was significantly lower in both patient groups (P < 0.001) compared to healthy controls. No correlation was observed between selenium and activities of selenoenzymes, tau, Aβ42 or tau-to-Aβ42 ratio, when analysing independently, indicating that blood selenium may not be directly involved in Aβ production and in regulating tau/Aβ42-mediated mechanism in AD. The present study emphasizes the enhanced oxidative stress in AD pathology and plasma tau and tau-to-amyloid ratio as possible markers to differentiate AD from VD. The study also points that blood selenium may not be involved in regulating oxidative stress in AD, and a longitudinal study correlating plasma and cerebrospinal fluid (CSF) selenium and selenoprotein levels is warranted.

Similar content being viewed by others
References
Shaji KS, Jotheeswaran AT, Girish N, et al (2010) Dementia India Report: prevalence, impact, costs and services for dementia. Alzheimer’s & Related Disorders Society of India. http://www.alzheimer.org.in/assets/dementia.pdf. Accessed 25 Aug 2013
Zawia NH, Lahiri DK, Cardozo-Pelaez F (2009) Epigenetics, oxidative stress and Alzheimer’s disease. Free Readic Biol Med 46:1241–1249
Padurariu M, Ciobica A, Hritcu L et al (2010) Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 469:6–10
Butterfield DA, Swomley AM, Sultana R (2013) Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal 19:823–835
Puertas MC, Martinez-Martos JM, Cobo MP et al (2012) Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp Gerontol 47:625–630
Casado A, Lopez-Fernandez ME, Casado MC et al (2008) Lipid peroxidation and antioxidant enzyme activities in vascular dementia and Alzheimer dementias. Neurochem Res 33:450–458
Naziroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Naziroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca2+ signalling and TRP channel activation in nervous system. J Recept Signal Transduct Res 32:134–141
Naziroğlu M, Yürekli VA (2013) Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 33:589–599
Gwon AR, Park JS, Park JH et al (2010) Selenium attenuates A beta production and A beta-induced neuronal death. Neurosci Lett 469:391–395
Steinbrenner H, Sies H (2013) Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys 536:152–157
Akbaraly NT, Hininger-Favier I, Carriere I et al (2007) Plasma selenium over time and cognitive decline in the elderly. Epidemiology 18:52–58
Loef M, Schrauzer GN, Walach H (2011) Selenium and Alzheimer’s disease: a systematic review. J Alzheimers Dis 26:81–104
Kapaki E, Paraskevas GP, Zalonis I et al (2003) CSF tau protein and beta-amyloid (1-42) in Alzheimer's disease diagnosis: discrimination from normal ageing and other dementias in the Greek population. Eur J Neurol 10:119–128
Mayeux R, Honig LS, Tang MX et al (2003) Plasma Aβ40 and Aβ42 and Alzheimer’s disease. Relation to age, mortality and risk. Neurology 61:1185–1190
Sparks DL, Kryscio RJ, Sabbagh MN et al (2012) Tau is reduced in AD plasma and validation of employed ELISA methods. Am J Neurodegener Dis 1:99–106
Sunderland T, Linker G, Mirza N et al (2003) Decreased β amyloid 1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer’s disease. JAMA 289:2094–2103
Rice-Evans C (1996) Free radicals and antioxidants in atherosclerosis. In: Blake D, Winyard PG (eds) Immunopharmacology of free radical species. Academic, London, pp 39–52
Folstein MF, Folstein SE, Mc Hugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Rani P, Unni M, Karthikeyan J (2004) Evaluation of antioxidant properties of berries. Indian J Clin Biochem 19:103–110
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Drabkin DL, Austin JH (1935) Spectrophotometric studies II. Preparations from washed blood cells; nitric oxide haemoglobin and sulfhaemoglobin. J Biol Chem 112:51–65
Safaralizadeh R, Kardar GA, Pourpak Z et al (2005) Serum concentrations of selenium in healthy individuals living in Tehran. Nutr J 4:32–35
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Arner ES, Zhong L, Holmgren A (1999) A preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol 300:226–239
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Bio 37:201–204
Calberg I, Mannervik J (1985) Glutathione reductase. Methods Enzymol 113:484–490
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Lobarzewski J, Ginalska G (1995) Industrial use of soluble or immobilized plant peroxidases. Plant peroxidase Newsletter 6:3–7
Balinsky D, Bernstein RR (1963) The purification and properties of glucose-6-phosphate dehydrogenase from human erythrocytes. Biochim Biophy Acta 67:313–315
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophy 82:70–77
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Roher AE, Esh CL, Kokjohn TA et al (2009) Aβ peptides in human plasma and their significance for Alzheimer’s disease. Alzheimers Dement 5:18–29
Toledo JB, Shaw LM, Trojanowski JQ (2013) Plasma amyloid beta measurements—a desired but elusive Alzheimer’s disease biomarker. Alzheimers Res Ther 5:8–17
Klapinska B, Poprezecki S, Danch A et al (2005) Blood selenium concentration of residents of upper Silesia: relation to age and gender. Polish J of Environ Stud 15:753–758
van Eersel J, Ke YD, Liu X et al (2010) Sodium selenate mitigates tau pathology, neurodegeneration and functional deficits in Alzheimer’s disease. Proc Natl Acad Sci U S A 107:13888–13893
Bellinger FP, He Q, Bellinger MT et al (2008) Association of selenoprotein P with Alzheimer’s pathology in human cortex. J Alzheimers Dis 15:465–472
Strozyk D, Launer LJ, Adlard PA et al (2009) Zinc and copper modulate Alzheimer Abeta levels in human cerebrospinal fluid. Neurobiol Aging 30:1069–1077
Naziroğlu M (2009) Role of selenium on calcium signalling and oxidative stress induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Gil L, Siems W, Mazurek B et al (2006) Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radial Res 40:495–505
Acknowledgments
The study was supported by the Indian Council of Medical Research (ICMR) and Department of Biotechnology (DBT), New Delhi. The authors thank Dr. M.B. Pranesh, PSG IMS&R, Coimbatore, and Dr. Jacob Roy, ARDSI, Thrissur, for referring patients for the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnan, S., Rani, P. Evaluation of Selenium, Redox Status and Their Association with Plasma Amyloid/Tau in Alzheimer’s Disease. Biol Trace Elem Res 158, 158–165 (2014). https://doi.org/10.1007/s12011-014-9930-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-9930-x