Abstract
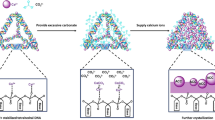
Bulk fabrication of ordered hollow structural particles (HSPs) with large surface area and high biocompatibility simultaneously is critical for the practical application of HSPs in biosensing and drug delivery. In this article, we describe a smart approach for batch synthesis of calcium carbonate nanotubes (CCNTs) based on supported liquid membrane (SLM) with large surface area, excellent structural stability, prominent biocompatibility, and acid degradability. The products were characterized by transmission electron micrograph, X-ray diffraction, Fourier transform infrared spectra, UV–vis spectroscopy, zeta potential, and particle size distribution. The results showed that the tube-like structure facilitated podophyllotoxin (PPT) diffusion into the cavity of hollow structure, and the drug loading and encapsulation efficiency of CCNTs for PPT are as high as 38.5 and 64.4 wt.%, respectively. In vitro drug release study showed that PPT was released from the CCNTs in a pH-controlled and time-dependent manner. The treatment of HEK 293T and SGC 7901 cells demonstrated that PPT-loaded CCNTs were less toxic to normal cells and more effective in antitumor potency compared with free drugs. In addition, PPT-loaded CCNTs also enhanced the apoptotic process on tumor cells compared with the free drugs. This study not only provides a new kind of biocompatible and pH-sensitive nanomaterial as the feasible drug container and carrier but more importantly establishes a facile approach to synthesize novel hollow structural particles on a large scale based on SLM technology.








Similar content being viewed by others
References
Luo B, Xu S, Luo A et al (2011) Mesoporous biocompatible and acid-degradable magnetic colloidal nanocrystal clusters with sustainable stability and high hydrophobic drug loading capacity. ACS Nano 5:1428–1435. doi:10.1021/nn103213y
Khajeh M (2010) Silver nanoparticles for the adsorption of manganese from biological samples. Biol Trace Elem Res 138:337–345. doi:10.1007/s12011-009-8600-x
Wang WR, Zhu RR, Xiao R et al (2011) The electrostatic interactions between nano-TiO2 and trypsin inhibit the enzyme activity and change the secondary structure of trypsin. Biol Trace Elem Res 142:435–446. doi:10.1007/s12011-010-8823-x
Zhang YZ, Li HR, Dai J et al (2010) Spectroscopic studies on the binding of cobalt (II) 1,10-phenanthroline complex to bovine serum albumin. Biol Trace Elem Res 135:136–152. doi:10.1007/s12011-009-8502-y
Au C, Mutkus L, Dobson A et al (2007) Effects of nanoparticles on the adhesion and cell viability on astrocytes. Biol Trace Elem Res 120:248–256. doi:10.1007/s12011-007-0067-z
Kong B, Zhu AW, Luo YP et al (2011) Sensitive and selective colorimetric visualization of cerebral dopamine based on double molecular recognition. Angew Chem Int Ed 50:1837–1840. doi:10.1002/anie.201007071
Allen TM, Cullis PR (2004) Drug delivery systems: entering the mainstream. Science 303:1818–1822. doi:10.1126/science.1095833
Arnold AM (1979) Podophyllotoxin derivative VP 16-213. Cancer Chemother Pharmacol 3:71–80. doi:10.1007/BF00254976
Tian X, Zhang FM, Lib WG (2002) Antitumor and antioxidant activity of spin labeled derivatives of podophyllotoxin (GP-1) and congeners. Life Sci 70:2433–2443
Gordaliza M, Castro MA, DelCorral JM et al (2000) Antitumor properties of podophyllotoxin and related compounds. Curr Pharm Des 6:1811–1839. doi:10.2174/1381612003398582
Van-maanen JMS, Retal J, De-vries J et al (1988) Mechanism of action of antitumor drug etoposide: a review. J Natl Cancer Inst 80:1526–1533. doi:10.1093/jnci/80.19.1526
Shah JC, Chen JR, Chow D (1989) Preformulation study of etoposide: identification of physicochemical characteristics responsible for the low and erratic oral bioavailability of etoposide. Pharm Res 6:408–412. doi:10.1023/A:1015935532725
Li A, Qin LL, Zhu D et al (2010) Signalling pathways involved in the activation of dendritic cells by layered double hydroxide nanoparticles. Biomaterials 31:748–756. doi:10.1016/j.biomaterials.2009.09.095
Li A, Qin LL, Wang WR et al (2011) The use of layered double hydroxides as DNA vaccine delivery vector for enhancement of anti-melanoma immune response. Biomaterials 2:469–477. doi:10.1016/j.biomaterials.2010.08.107
Xiao R, Wang WR, Pan LL et al (2011) A sustained folic acid release system based on ternary magnesium/zinc/aluminum layered double hydroxides. J Mater Sci 46:2635–2643. doi:10.1007/s10853-010-5118-8
Qin LL, Xue M, Wang WR et al (2010) The in vitro and in vivo anti-tumor effect of layered double hydroxides nanoparticles as delivery for podophyllotoxin. Int J Pharm 388:223–230. doi:10.1016/j.ijpharm.2009.12.044
Zhu RR, Qin LL, Wang M et al (2009) Preparation, characterization and anti-tumor property of podophyllotoxin-loaded solid lipid nanoparticles. Nanotechnology 20:055702 (7 pp). doi:10.1088/0957-4484/20/5/055702
Qin LL, Wang SL, Zhang R et al (2008) Two different approaches to synthesizing Mg–Al-layered double hydroxides as folic acid carriers. J Phys Chem Solids 69:2779–2784. doi:10.1016/j.jpcs.2008.06.144
Lee Y, Lee J, Bae CJ, Park JG, Noh HJ, Park JH, Hyeon T (2005) Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv Funct Mater 15:503–509. doi:10.1002/adfm.200400187
Dolinska B, Mikulska A, Caban A et al (1998) A model for calcium permeation into small intestine. Biol Trace Elem Res 142:456–464. doi:10.1007/s12011-010-8827-6
Helmlinger G, Yuan F, Dellian M, Jain RK (1997) Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 3:177–182. doi:10.1038/nm0297-177
Wei W, Ma GH, Hu G et al (2008) Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. J Am Chem Soc 130:15808–15810. doi:10.1021/ja8039585
Lee Y, Lee J, Bae CJ et al (2005) Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Ad Funct Mater 15:503–509. doi:10.1002/adfm.200400187
Han S, Jang B, Oh SM et al (2005) Simple synthesis of hollow tin dioxide microspheres and their application to lithium-ion battery anodes. Ad Funct Mater 15:1845–1850. doi:10.1002/adfm.200500243
Caruso F, Caruso RA, Mohwald H (1998) Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 282:1111–1114. doi:10.1126/science.282.5391.1111
Loges N, Graf K, Nasdala L et al (2006) Probing cooperative interactions of tailor-made nucleation surfaces and macromolecules: a bioinspired route to hollow micrometer-sized calcium carbonate particles. Langmuir 22:3073–3080. doi:10.1021/la0528596
Suzuki M, Nagasawa H, Kogure T (2006) Synthesis and structure of hollow calcite particles. Cryst Growth Des 6:2004–2006. doi:10.1021/cg0602921
Sugihara H, Inoue K, Nakayama M et al (2009) A novel nanotube of composite of calcium carbonate and calcium sulfate. Mater Lett 63:322–324. doi:10.1016/j.matlet.2008.10.025
Yu H, Yu J, Liu S et al (2007) Template-free hydrothermal synthesis of CuO/Cu2O composite hollow microspheres. Chem Mater 19:4327–4334. doi:10.1021/cm070386d
Sun DM, Zhu DZ (2009) Bi-template effect of a vegetal system on the synthesis of alkaline-earth tungstate nanocrystals. J Mater Res 24:347–351. doi:10.1557/JMR.2009.0072
Sun DM, Wu QS (2006) A novel method for crystal control: synthesis and design of strontium carbonate with different morphologies by supported liquid membrane. J Appl Crystallogr 39:544–549. doi:10.1107/S0021889806015925
Wu QS, Sun DM, Liu HJ et al (2004) Abnormal polymorph conversion of calcium carbonate and nano-self-assembly of vaterite by a supported liquid membrane system. Cryst Growth Des 4:717–720. doi:10.1021/cg034247u
Sun DM, Zhu DZ (2008) Synthesis and design of MnCO3 crystals with different morphologies by supported liquid membrane. J Chem Crystallogr 38:949–952. doi:10.1007/s10870-008-9419-6
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Method 65:55–63. doi:10.1016/0022-1759(83)90303-4
Addadi L, Weiner S (1992) Control and design principles in biological mineralization. Angew Chem Int Ed 31:153–169. doi:10.1002/anie.199201531
Naka K, Keum DK, Tanaka Y et al (2000) Control of crystal polymorphs by a ‘latent inductor’: crystallization of calcium carbonate in conjunction with in situ radical polymerization of sodium acrylate in aqueous solution. J Chem Soc 16:1537–1538. doi:10.1039/b004649n
Mann S (2001) Biomineralization. Principles and concepts in bioinorganic materials chemistry. Oxford University Press, New York, p 104
Smith BL, Schaffer TE, Viani M et al (1999) Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature 399:761–763. doi:10.1038/21607
Sangwal K (1996) Growth kinetics and surface morphology of crystals grown from solutions: recent observations and their interpretations. Prog Cryst Growth Charact 36:163–248. doi:10.1016/S0960-8974(98)00009-6
Mann S, Didymus JM, Sanderson NP et al (1990) Morphological influence of functionalized and non-functionalized α, ω-dicarboxylates on calcite crystallization. J Chem Soc Faraday Trans 86:1873–1880. doi:10.1039/FT9908601873
Didymus JM, Oliver P, Mann S (1993) Influence of low-molecular-weight and macromolecular organic additives on the morphology of calcium carbonate. J Chem Soc Faraday Trans 89:2891–2900. doi:10.1039/FT9938902891
Shivkumara C, Singh P, Gupta A et al (2006) Synthesis of vaterite CaCO3 by direct precipitation using glycine and L-alanine as directing agents. Mater Res Bull 41:1455–1460. doi:10.1016/j.materresbull.2006.01.026
Nan ZD, Chen XN, Yang QQ et al (2008) Structure transition from aragonite to vaterite and calcite by the assistance of SDBS. J Colloid Interface Sci 325:331–336. doi:10.1016/j.jcis.2008.05.045
Lee MK, Lim SJ, Kim CK (2007) Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials 28:2137–2146. doi:10.1016/j.biomaterials.2007.01.014
Roberts JE, Wielgus AR, Boyes WK et al (2008) Phototoxicity and cytotoxicity of fullerol in human lens epithelial cell. Toxicol Appl Pharmacol 228:49–58. doi:10.1016/j.taap.2009.09.021
Choy JH, Jung JS, Oh JM (2004) Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 25:3059–3064. doi:10.1016/j.biomaterials.2003.09.083
Acknowledgments
This work was financially supported by the Major State Basic Research Development Program of China (973 Program, grant no. 2010CB933901), the National Natural Science Foundation of China (grant no. 50802063, 31140038), the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20090072120019), Science and Technology Commission of Shanghai Municipality (grant no. 11411951500), the Fundamental Research Funds for the Central Universities, and Young Excellent Talents Plans in Tongji University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Tang and Dong-Mei Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tang, J., Sun, DM., Qian, WY. et al. One-Step Bulk Preparation of Calcium Carbonate Nanotubes and Its Application in Anticancer Drug Delivery. Biol Trace Elem Res 147, 408–417 (2012). https://doi.org/10.1007/s12011-012-9325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9325-9