Abstract
An in vitro model was used to simulate the intestinal permeation of calcium ions depending on the type of salt (carbonate, fumarate, citrate, or gluconate), its concentration (1.0, 2.5, 5.0, or 10 mM/l), and pH (1.3, 4.2, 6.2, or 7.5). To simulate the conditions for calcium permeation in a patient in a fasting state, the solutions were placed in contact with segments of small intestine of pig: stomach, duodenum, jejunum, and ileum. The percent permeation, its rate, and half-time were measured in each case. In all cases, the maximum permeation was seen at 1 mM concentration, depending on pH: 100% for carbonate at pH 1.3; 82% for fumarate, pH 6.2; 79.5% for citrate at pH 4.2, and 81% for gluconate at pH 7.4. The maximum rate of permeation (% h−1) was also observed at 1 mM: 2.16 for carbonate at pH 1.3, 0.29 for fumarate at pH 6.2, 0.26 for citrate at pH 4.2, and 0.28 for gluconate at pH 7.4. The shortest half-time permeation (t 1/2, h) for 1 mM solutions depended also on pH (in parentheses): carbonate 0.3 (1.3), fumarate 2.4 (6.2), citrate 2.6 (4.2), and gluconate 2.5 (7.4). The results suggest that calcium carbonate and citrate can be recommended to patients with normal gastric acidity and hyperacidity while fumarate and gluconate to patients with hypoacidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insufficient dietary calcium intake often leads to systemic and cellular disorders of calcium homeostasis [1–4]. To correct calcium deficiency, supplements are given in doses of 500–1,000 mg elemental calcium as inorganic and organic salts [1, 5]. Common forms of oral administration of calcium include calcium carbonate, gluconate, lactogluconate, glucolactobionate, dobesilate, citrate, lactate, and others [6]. The calcium content differs in various salts. Among these salts, calcium carbonate is more often used because it contains the highest amount of elemental calcium ∼40%.
However, despite the large variety of calcium preparations available for calcium supplementation, their effectiveness is inadequate because of poor absorption after oral administration [6]. To make calcium absorption more effective, researchers look for substances that enhance its gastrointestinal absorption [3, 7]. To learn how much substance passes through GI cell membranes after oral administration, both in vitro models using Franz diffusion cells and in vivo models are used in absorption studies [8–10].
The purpose of this study was to determine the degree and half-time of Ca(II) ion permeation depending on the type of calcium salt (carbonate, fumarate, citrate, or gluconate), its concentration (1, 2.5, 5, or 10 mmol/l), and pH of the medium (1.3, 4.2, 6.2, or 7.5). The organic salts are commonly used in supplementation protocols [1, 6]. Calcium carbonate was used as reference.
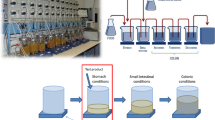
The permeation of Ca(II) ions at these concentrations and pH was measured using swine small intestine. Tested ions passed from the donor environment (stomach) to the acceptor environment, which corresponded to the natural condition of different parts of the GI tract (stomach, duodenum, jejunum, or ileum). An in vitro model was applied to study the permeation process. The results were used to estimate the permeation of the different solutions tested after oral administration to a patient in fasting state, where gastric pH is about 1.3 [11].
Materials and Methods
Reagents
The purity of all reagents was 99.9–99.99%, i.e., contaminants cannot be detected by conventional methods of analysis. Calcium carbonate (CaCO3) was purchased from POCH, Gliwice, Poland; calcium fumarate (CaC4H2O4·3H2O) was obtained from FZNP Biochefa, Sosnowiec, Poland; calcium gluconate (Ca[C6H11O7]2·H2O) was from Pharma Cosmetic, Kraków, Poland; and calcium citrate Ca3[C6H5O7]2·4H2O was from Sigma-Aldrich Chemie GmbH, Buchs, USA. Some characteristics of these salts are shown in Table 1.
Small Intestine
Fifteen specimens of small intestine were collected from 6-month-old pigs weighing 100 ± 2 kg. The samples weighed on average 290 ± 10 mg. They were handled and stored in accordance with the methodology of maintenance of organs and tissues intended for transplantation [12]. Once removed from the carcass and dissected, the intestines were washed with 0.9% NaCl solution to an absorbance value with an extinction coefficient ε < 0.02 at 278 nm, and then quickly frozen at −20°C until needed for the experiments.
Body Fluids
Body fluids simulating the natural conditions of certain gastrointestinal sections were used: artificial gastric juice containing pepsin at pH 1.3 to simulate stomach, artificial intestinal liquids at pH 4.2 and 6.2 containing pepsin and pancreatin to simulate the duodenal and jejunal environments, and artificial intestinal fluid at pH 7.5 containing pancreatin to imitate the ileal environment [11].
Determination of Calcium Ions
For the quantitative determination of Ca(II), a UV–Vis spectrometer (Marcel Media, France) was used following a validated method [13].
Research Model
The experimental model consisted of a standard Franz diffusion cell [8]. The cell consisted of two chambers holding 2 ml each. One acted as the donor chamber (donor compartment, D) and the other as the acceptor chamber (acceptor compartment, A). The two chambers were kept at the same level and were separated by the small intestine tissue to be tested. Chamber D was filled with 2 ml of the artificial gastric juice in which a calcium salt was dissolved (carbonate, fumarate, citrate, or gluconate) at different concentrations (1, 2.5, 5, or 10 mmol/l). Compartment A was filled with 2 ml of the appropriate fluid simulating different sections of the small intestine at pH 1.3, 4.2, 6.2, or 7.5.
After 0, 0.25, 0.50, 0.75, 1, 2, 3, 4, 5, and 6 h, the entire amount of liquid was collected from A and the absorbance was read at 570 nm to determine the amount of Ca(II). The experiment was designed following a Latin square 4 × 3 scheme [14]. The plan of the experiment is presented in Table 2.
The process of Ca(II) penetration was traced according to the type of calcium salts: a1—carbonate, a2—fumarate; a3—citrate, or a4—gluconate; the calcium concentration: b1—1 mM, b2—2.5 mM, b3—5 mM, or b4—10 mM; and the pH of the acceptor environment: c1—1.3, c2—4.2, c3—6.2, or c4—7.5.
Ions penetrated following first-order kinetics. The ion transfer rate constant (k) was calculated by means of the following formula:
where C 1 is the total Ca(II) in A at time t 1 = 0 h, and C 2 is the total Ca(II) in A at t 2 = 6 h.
From the rate constant, the half-time of penetration is given by:
Statistical Analyses
The results are presented as the mean (x) of ten samples. The standard deviation (SD) and correlation coefficients (r 2) were calculated. Correlations were calculated between calcium concentration and acceptor environment pH as well as the degree and half-time of Ca(II) penetration. The Student t test was used to establish statistical significance, set at p < 0.05. The software packages Excel (Microsoft) and Statistic for Windows 5.1 (StatSoft Inc.) were used for all calculations.
Results and Discussion
The experiments were designed to simulate the conditions under which calcium salts are administered orally on an empty stomach. In such case, the gastric pH is about 1.3 [11]. Calcium ion solutions passed from the chamber mimicking the stomach to other simulating different parts of the gastrointestinal tract under conditions that are dependent on the chemical form of calcium, its concentration, and the pH in the acceptor chamber.
The degree of ion migration from D to A was varied: 9.6–100% for carbonate, 18.3–81.2% for fumarate, 17.8–79.5% for citrate, and 21.2–81.0% for gluconate. One hundred percent penetration is seen for 1 mM calcium carbonate. Increasing the concentration 10-fold caused a near 10-fold decrease (9.6%) of the degree of penetration. The effect of concentration on ion penetration and correlations (r 2) are given in Fig. 1.
The degree of ion penetration for the organic forms of calcium was significantly different as measured in relation to calcium carbonate solutions, with the exception of 5 mM fumarate. The lower the calcium concentration, the greater degree of ion penetration: calcium citrate (r 2 = −0.908), carbonate (r 2 = −0.821), and fumarate (r 2 = −0.811). No such significant dependence was observed in case of calcium gluconate solutions (r 2 = −0.368).
These in vitro results are consistent with previously published data on the effects of dose and molecular mass of the calcium salt anion: fumarate, gluconate, and chloride on the absorption of Ca(II) ions. The amount of absorbed Ca(II) was not associated with the molecular mass of the anion [15].
The degree of calcium absorption in vivo, however, depends primarily on the needs of the body. It was found that at the age of 25, absorption is reduced to 20–30%, and after 35 years of age, it drops down to 15%. In patients with osteoporosis, the body’s requirement for calcium increases significantly [1, 4, 16].
All tested substances were salts of weak acid and strong base, and their highest concentration was 10-fold lower than the concentration of hydrochloric acid in the stomach, conditions ensuring total ionization (α = 100%).
Figure 2 presents the effect of acceptor environment pH on ion penetration and correlations (r 2). Interestingly, the degree of Ca(II) penetration at pH 1.3 was not the greatest and differed significantly depending on the type of salt. Only the Ca(II) ions from calcium carbonate penetrated completely into an environment of pH 1.3. Under these conditions, penetration was 57.2% ions from citrate, 39.7% from gluconate, and 18.3% from calcium fumarate.
Calcium carbonate is not soluble in water, but in contact with gastric hydrochloric acid, it changes into the chloride becoming freely soluble and best absorbed [7]. This result suggests that calcium carbonate could be administered on an empty stomach or before meals. Calcium from carbonate did not pass in near neutral to alkaline media.
The environment with a pH of 4.2 favored 79.8% penetration of calcium from citrate, while more neutral conditions favored ions from fumarate (82%) and gluconate (81%).
Penetration was directly correlated to acidity of the acceptor environment in the cases of carbonate (r = −0.987) and citrate (r = −0.654), and inversely for fumarate (r = 0.900) and gluconate (r = 0.412), suggesting that calcium citrate could be used in patients with hyperacidity [1.17]. Calcium in citrate is bound to a weak organic acid, which favors absorption. The amount of ionized calcium remains stable for several hours even after pancreatic juice secretion [7].
The half-time of permeation of the different solutions tested t 1/2 and the respective correlations are shown in Fig. 3. The correlation was directly dependent on the concentration for carbonate (r 2 = 0.995), citrate (r 2 = 0.988), and fumarate (r 2 = 0.711) but not for gluconate (r 2 = 0.023).
Calcium carbonate is one of the most commonly administered calcium salts due to the highest element content in the molecule ∼40%. If administered in a concentration of 1 mM (40 mg of calcium), 20 mg ions would pass within 30 min. Our results show that the longest penetration time (41.3 h) was recorded for 10 mM calcium carbonate. Thus, 10 mM calcium carbonate (400 mg elemental calcium) would result on the absorption of only 200 mg of this element in about 41 h. What this means is that the oral administration of 400 mg calcium (as carbonate) in a fasting patient would result in stomach retention of the element, limiting its use as supplement. It is then preferable to administer the salt in smaller doses. Patients, however, usually prefer to take the entire daily ration of the supplement in a single dose, which would be of little help.
In view of this, it seems that it would be much more effective to administer calcium gluconate or fumarate. The Ca(II) penetration half-time from 10 mM solutions of these salts was 8.22 and 15.68 h, respectively, five and 2.6 times shorter in comparison with calcium carbonate. It should be noted, however, that the calcium content in gluconate is only ∼8.9%. Thus, supplementation with this salt should be in larger doses as compared with other calcium salts tested.
To provide 500 mg of calcium, a patient should take up to 5.6 g of calcium gluconate, 2.4 g citrate, 2.6 g of fumarate, and only 1.25 g of calcium carbonate. This makes calcium fumarate an interesting choice [15–17].
Its effectiveness and tolerance were determined in the fight against calcium and phosphorus disturbances in patients on dialysis due to chronic renal failure compared with treatment with calcium carbonate [16]. To obtain a similar metabolic compensating effect of calcium and phosphorus levels in such patients, it would be sufficient to use 30% less calcium as fumarate than carbonate without noticeable side effects. Positive effects of calcium fumarate were also found when it was administered to female rats with experimentally induced osteoporosis [16]. In rats, the absorption of calcium fumarate is comparable with other calcium salts [18, 19].
The effect of pH on Ca(II) ion penetration half-time in the acceptor environment is shown in Fig. 4. The parameters of penetration from selected salts depending on the conditions are presented in Table 3.
Higher pH in the acceptor environment favored a longer time for calcium ions to migrate from its solutions: carbonate (r 2 = 0.927) and citrate (r 2 = 0.682). With a higher pH, ions from fumarate (r 2 = −0.951) more rapidly penetrated into the acceptor. No significant relationship was observed in case of calcium gluconate (r 2 = −0.055). These results indicate that calcium from carbonate and citrate is well absorbed in patients with normal stomach acidity and hyperacidity, while supplementation with fumarate and gluconate will be more effective in patients with hypoacidity.
Conclusion
On the basis of in vitro studies, we can draw conclusions on the absorption of the tested calcium salts after oral administration in a fasting state [10]. Taking into account the degree of Ca(II) penetration, the examined salts can be classified into groups of substances of high (>90%), medium (50–89%), or poor absorption (<50%). Calcium carbonate will be well absorbed at a concentration of 1 mM (40 mg calcium) at pH 1.3. Substances with medium absorption would include calcium citrate also at 1 and at 2.5 mM (40 and 100 mg of calcium, respectively) at pH values of 4.2 and 1.3; calcium fumarate at pH 6.2 and 7.4 and gluconate in an environment with a pH value of 7.4.
The degree and half-time of Ca(II) penetration depend on the type of calcium salt, its concentration, and acceptor pH. During oral supplementation with calcium salts, they should be administered at low doses to make them more accessible to the organism. Suitable conditions for absorption of calcium salts may provide higher bioavailability and thus higher treatment efficacy during supplementation. Calcium from carbonate and citrate should be used in patients with normal stomach acidity and hyperacidity, while calcium fumarate and gluconate should be recommended for supplementation in patients with hypoacidity.
References
Straub DA (2007) Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract 3:286–296
Ramasamy I (2006) Recent advances in physiological calcium homeostasis. Clin Chem Lab Med 3:237–273
Buzinaro EF, Almeida RN, Mazeto GM (2006) Bioavailability of dietary calcium. Arq Bras Endocrinol Metabol 5:852–861
Ohta A (2006) Prevention of osteoporosis by foods and dietary supplements. The effect of fructooligosaccharides (FOS) on the calcium absorption and bone. Clin Calcium 10:1639–1645
Weaver CM, Rothwell AP, Wood KV (2006) Measuring calcium absorption and utilization in humans. Curr Opin Clin Nutr Metab Care 5:568–574
Hanzlik RP, Fowler SC, Fisher DH (2005) Relative bioavailability of calcium from calcium formate, calcium citrate and calcium carbonate. J Pharmacol Exp Ther 313:1217–1222
Dolinska B, Mikulska A, Ryszka F (2009) Factors enhancing calcium’s absorption. Ann Acad Med Siles 1:89–96
Franz TJ (1975) Percutaneus absorption and the relevance of in vitro data. J Invest Dermatol 64:190–195
Yee S (1997) In vitro permeability across Cacao-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—fact or myth. Pharm Res 14:763–766
Dolińska B, Mikulska A, Ostróżka-Cieślik A, Ryszka F (2010) The influence of condition on permeation of Ca(II) ions from solutions of selected calcium’s salts through model membrane. Biol Trace Elem Res. doi:10.1007/s12011-010-8675-4
Dressman JB, Amidon GL, Reppas C (1998) Dissolution testing as a prognostic tool for oral absorption: immediate release dosage forms. Pharm Res 15:11–22
Toledo-Pereyra LH, Lopez-Neblina F, Toledo A (2010) Organ freezing. In: Toledo-Pereyra LH (ed) Organ preservation for transplantation, 3rd edn. Landes Bioscience, Austin, http://www.landesbioscience.com/books/iu/id/2258
Nowatzke W, Woolf E (2007) Best practices during bioanalytical method validation for the characterization of assay reagents and the evaluation of analyte stability in assay standards, quality controls, and study samples. AAPS J 9:E117–E122, serial online
Kafarow VV (1979) Cybernetics methods in chemistry and chemical technology. WNT, Warsaw
Ryszka F, Gadomska-Nowak M, Krupej J, Dolinska B (1997) The effect of calcium dose and relative molecular mass on Ca2+-ions absorption in the small intestine. Acta Pharm Croatica 4:275–279
Ryszka F, Dolińska B, Suszka-Switek A (2003) Calcium concentration at rat females with osteoporosis after applying calcium fumarate. Boll Chim Farm 7:258–259
Kokot M, Adamczyk M, Ryszka F (1999) Assessment of efficiacy and tolerance Biohical 250 in haemodialysed patients with end stage renal failure. Nefrol Dializoterapia Pol 3:125–128
Weaver CM, Martin BR (2003) Absorption of calcium fumarate salts is equivalent to other calcium salts when measured in the rat model. J Agric Food Chem 17:4974–4975
Dolinska B, Mikulska A, Ryszka F (2008) Calcium preparation effeciveness in prevention of acalcerosis. Farm Przegląd Naukowy 7–8:5–8
Acknowledgement
The research was with funds for a project co-financed by the European Regional Development Foundation as a part of the Operating Program of Innovative Economy No. UDA-POIG.01.03.1-00-133/08-00 “Innovative technologies of the production of bio-preparations on the basis of new generation of eggs” in the years 2009–2011.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dolinska, B., Mikulska, A., Caban, A. et al. A Model for Calcium Permeation into Small Intestine. Biol Trace Elem Res 142, 456–464 (2011). https://doi.org/10.1007/s12011-010-8827-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8827-6