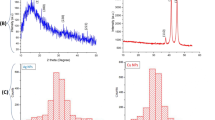
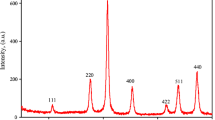
Abstract
In this study, silver nanoparticles were prepared and used for separation and preconcentration of manganese from biological samples. The technical feasibility of silver nanoparticles for manganese removal was investigated under batch studies. The effects of different parameters such as pH of solution, time (t), amounts of PAN (E), and silver nanoparticles (N) on the adsorption of manganese by silver nanoparticle were investigated using factorial design and response surface methodology based on Box–Behnken design. Thermodynamic parameters indicate the adsorption process to be exothermic. The limit of detection of the proposed method followed by inductively coupled plasma was found to be 0.08 µg L−1. The method was applied to determine of manganese in biological samples.



Similar content being viewed by others
References
Underwood EJ (1997) Trace elements in human and animal nutrition. Academic, New York, p 170
Soko L, Chimuka L, Cukrowska E, Pole S (2003) Extraction and preconcentration of manganese (II) from biological fluids (water, milk and blood serum) using supported liquid membrane and membrane probe methods. Anal Chim Acta 485:25–35
Rajic N, Stojakovic D, Jevtic S, Logar NZ, Kovac J, Kaucic V (2009) Removal of aqueous manganese using the natural zeolitic tuff from the Vranjska Banja deposit in Serbia. J Hazard Mater 172:1450–1457
Shamsipur T, Mostafavi A (2009) Application of modified multiwalled carbon nanotubes as a sorbent for simultaneous separation and preconcentration trace amounts of Au(III) and Mn(II). J Hazard Mater 168:1543–1553
Duran A, Soylak M, Tuncel SA (2008) Poly(vinyl pyridine-poly ethylene glycol methacrylate-ethylene glycol dimethacrylate) beads for heavy metal removal. J Hazard Mater 155:114–120
Kaminski MD, Nunez L, Visser AE (1999) Evaluation of extractant-coated ferromagnetic microparticles for the recovery of hazardous metals from waste solution. Sep Sci Technol 34:1103–1120
Montgomery DC (1997) Design and analysis of experiments, 4th edn. Wiley, New York
Box GEP, Hunter WG, Hunter JS (1997) Statistics for experimenters. Wiley, New York
Ferreira HS, Santos ACN, Portugal LA, Costa ACS (2008) Pre-concentration procedure for determination of copper and zinc in food samples by sequential multi-element flame atomic absorption spectrometry. Talanta 77:73–76
Nazari F, Ebrahimi SN, Talebi M, Rassouli A, Bijanzadeh HR (2007) Multivariate optimisation of microwave assisted extraction of capsaicin from Capsicum frutescens L. and quantitative analysis by 1H-NMR. Phytochem Anal 18:333–340
Buco S, Morgagues M, Sergent M, Doumenq P, Mille G (2007) An experimental design approach for optimizing polycyclic aromatic hydrocarbon analysis in contaminated soil by pyrolyser-gas chromatography-mass spectrometry. Environ Res 104:209–215
Sivakumar M, Annadurai G, Mohan D (1999) Studies on Box-Behnken design experiments: cellulose acetate-polyurethane ultrafiltration membranes. Bioprocess Eng 21:65–68
Santelli RE, Bezerra MA, SantAna OD, Cassella RJ, Ferreira SLC (2006) Multivariate technique for optimization of digestion procedure by focussed microwave system for determination of Mn, Zn and Fe in food samples using FAAS. Talanta 68:1083–1088
Santos WLD, Santos CMMD, Costa JLO, Andrade HMC, Ferreira SLC (2004) Multivariate optimization and validation studies in on-line pre-concentration system for lead determination in drinking water and saline waste from oil refinery. Microchem J 77:123–129
Kumar A, Prasad B, Mishra IM (2008) Optimization of process parameters for acrylonitrile removal by a low-cost adsorbent using Box-Behnken design. J Hazard Mater 150:174–182
Segurola J, Allen NS, Edge M, Mahon AM (1999) Design of eutectic photoinitiator blends for UV/curable acrylated printing inks and coating. Prog Org Coat 37:23–37
Doroschuk VO, Lelyushok SO, Ishchenko VB, Kulichenko SA (2004) Flame atomic absorption determination of manganese(II) in natural water after cloud point extraction. Talanta 64:853–856
Lemos VA, Baliza PX, Carvalha ALD, Oliveria RV, Teixeira LSG, Bezerra MA (2008) Development of a new sequential injection in-line cloud point extraction system for flame atomic absorption spectrometric determination of manganese in food samples. Talanta 77:388–393
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khajeh, M. Silver Nanoparticles for the Adsorption of Manganese from Biological Samples. Biol Trace Elem Res 138, 337–345 (2010). https://doi.org/10.1007/s12011-009-8600-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8600-x