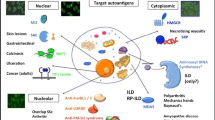
Abstract
Purpose of review
Dermatomyositis is an idiopathic inflammatory myopathy with a variety of systemic and cutaneous manifestations. The myositis-specific autoantibodies (MSAs) are associated with phenotypic features and provide a tool for sub-classification of dermatomyositis patients. This review focuses on recent work characterizing the clinical features that accompany the MSAs in dermatomyositis.
Recent findings
There is increasing recognition of the distinct clinical and pathological phenotypes associated with each MSA. Most of these features display considerable overlap between MSA groups. Despite this, there are notable differences between the typical combinations of cutaneous and systemic manifestations, response to therapy, prognosis, and disease sequelae that define each dermatomyositis MSA group.
Summary
The MSAs may ultimately improve diagnosis and sub-classification of dermatomyositis patients. However, more work is needed to understand the pathologic basis for much of the heterogeneity found within these subgroups.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344–7. https://doi.org/10.1056/NEJM197502132920706.
Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292(8):403–7. https://doi.org/10.1056/NEJM197502202920807.
Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76(12):1955–64. https://doi.org/10.1136/annrheumdis-2017-211468.
Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57(5):375–81. https://doi.org/10.4103/0019-5154.100486.
Targoff IN, Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985;28(7):796–803.
Srivastava P, Dwivedi S, Misra R. Myositis-specific and myositis-associated autoantibodies in Indian patients with inflammatory myositis. Rheumatol Int. 2016;36(7):935–43. https://doi.org/10.1007/s00296-016-3494-3.
Rider LG, Shah M, Mamyrova G, Huber AM, Rice MM, Targoff IN, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore). 2013;92(4):223–43. https://doi.org/10.1097/MD.0b013e31829d08f9.
Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med. 2016;280(1):8–23. https://doi.org/10.1111/joim.12451.
Shah M, Mamyrova G, Targoff IN, Huber AM, Malley JD, Rice MM, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore). 2013;92(1):25–41. https://doi.org/10.1097/MD.0b013e31827f264d.
Brouwer R, Hengstman GJ, Vree Egberts W, Ehrfeld H, Bozic B, Ghirardello A, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis. 2001;60(2):116–23.
Cruellas MG, Viana Vdos S, Levy-Neto M, Souza FH, Shinjo SK. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics (Sao Paulo). 2013;68(7):909–14. https://doi.org/10.6061/clinics/2013(07)04.
Selva-O'Callaghan A, Labrador-Horrillo M, Solans-Laque R, Simeon-Aznar CP, Martinez-Gomez X, Vilardell-Tarres M. Myositis-specific and myositis-associated antibodies in a series of eighty-eight Mediterranean patients with idiopathic inflammatory myopathy. Arthritis Rheum. 2006;55(5):791–8. https://doi.org/10.1002/art.22237.
Hamaguchi Y, Kuwana M, Hoshino K, Hasegawa M, Kaji K, Matsushita T, et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol. 2011;147(4):391–8. https://doi.org/10.1001/archdermatol.2011.52.
Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70(6):360–74.
O'Hanlon TP, Carrick DM, Targoff IN, Arnett FC, Reveille JD, Carrington M, et al. Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies: distinct HLA-A, -B, -Cw, -DRB1, and -DQA1 allelic profiles distinguish European American patients with different myositis autoantibodies. Medicine (Baltimore). 2006;85(2):111–27. https://doi.org/10.1097/01.md.0000217525.82287.eb.
O'Hanlon TP, Rider LG, Mamyrova G, Targoff IN, Arnett FC, Reveille JD, et al. HLA polymorphisms in African Americans with idiopathic inflammatory myopathy: allelic profiles distinguish patients with different clinical phenotypes and myositis autoantibodies. Arthritis Rheum. 2006;54(11):3670–81. https://doi.org/10.1002/art.22205.
Mierau R, Dick T, Bartz-Bazzanella P, Keller E, Albert ED, Genth E. Strong association of dermatomyositis-specific Mi-2 autoantibodies with a tryptophan at position 9 of the HLA-DR beta chain. Arthritis Rheum. 1996;39(5):868–76.
Shamim EA, Rider LG, Pandey JP, O'Hanlon TP, Jara LJ, Samayoa EA, et al. Differences in idiopathic inflammatory myopathy phenotypes and genotypes between Mesoamerican Mestizos and North American Caucasians: ethnogeographic influences in the genetics and clinical expression of myositis. Arthritis Rheum. 2002;46(7):1885–93. https://doi.org/10.1002/art.10358.
Love LA, Weinberg CR, McConnaughey DR, Oddis CV, Medsger TA Jr, Reveille JD, et al. Ultraviolet radiation intensity predicts the relative distribution of dermatomyositis and anti-Mi-2 autoantibodies in women. Arthritis Rheum. 2009;60(8):2499–504. https://doi.org/10.1002/art.24702.
Petri MH, Satoh M, Martin-Marquez BT, Vargas-Ramirez R, Jara LJ, Saavedra MA, et al. Implications in the difference of anti-Mi-2 and -p155/140 autoantibody prevalence in two dermatomyositis cohorts from Mexico City and Guadalajara. Arthritis Res Ther. 2013;15(2):R48. https://doi.org/10.1186/ar4207.
Komura K, Fujimoto M, Matsushita T, Kaji K, Kondo M, Hirano T, et al. Prevalence and clinical characteristics of anti-Mi-2 antibodies in Japanese patients with dermatomyositis. J Dermatol Sci. 2005;40(3):215–7. https://doi.org/10.1016/j.jdermsci.2005.09.004.
Mammen AL, Casciola-Rosen LA, Hall JC, Christopher-Stine L, Corse AM, Rosen A. Expression of the dermatomyositis autoantigen Mi-2 in regenerating muscle. Arthritis Rheum. 2009;60(12):3784–93. https://doi.org/10.1002/art.24977.
• Deakin CT, Yasin SA, Simou S, Arnold KA, Tansley SL, Betteridge ZE, et al. Muscle biopsy findings in combination with myositis-specific autoantibodies aid prediction of outcomes in juvenile dermatomyositis. Arthritis Rheumatol. 2016;68(11):2806–16. https://doi.org/10.1002/art.39753. First study to show that degree of severity of muscle biopsy, in combination with MSA subtype, is predictive of increased treatment duration in JDM.
• Pinal-Fernandez I, Casciola-Rosen LA, Christopher-Stine L, Corse AM, Mammen AL. The prevalence of individual histopathologic features varies according to autoantibody status in muscle biopsies from patients with dermatomyositis. J Rheumatol. 2015;42(8):1448–54. First paper to systematically analyze muscle biopsies in adult DM and identify histology features associated with specific MSAs.
Hengstman GJ, Vree Egberts WT, Seelig HP, Lundberg IE, Moutsopoulos HM, Doria A, et al. Clinical characteristics of patients with myositis and autoantibodies to different fragments of the Mi-2 beta antigen. Ann Rheum Dis. 2006;65(2):242–5. https://doi.org/10.1136/ard.2005.040717.
Reed AM, Crowson CS, Hein M, de Padilla CL, Olazagasti JM, Aggarwal R, et al. Biologic predictors of clinical improvement in rituximab-treated refractory myositis. BMC Musculoskelet Disord. 2015;16:257. https://doi.org/10.1186/s12891-015-0710-3.
Aggarwal R, Bandos A, Reed AM, Ascherman DP, Barohn RJ, Feldman BM, et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66(3):740–9. https://doi.org/10.1002/art.38270.
Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford). 2010;49(3):433–40. https://doi.org/10.1093/rheumatology/kep375.
Cao H, Pan M, Kang Y, Xia Q, Li X, Zhao X, et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res (Hoboken). 2012;64(10):1602–10. https://doi.org/10.1002/acr.21728.
Chen Z, Hu W, Wang Y, Guo Z, Sun L, Kuwana M. Distinct profiles of myositis-specific autoantibodies in Chinese and Japanese patients with polymyositis/dermatomyositis. Clin Rheumatol. 2015;34(9):1627–31. https://doi.org/10.1007/s10067-015-2935-9.
Bodoki L, Nagy-Vincze M, Griger Z, Betteridge Z, Szollosi L, Danko K. Four dermatomyositis-specific autoantibodies-anti-TIF1gamma, anti-NXP2, anti-SAE and anti-MDA5-in adult and juvenile patients with idiopathic inflammatory myopathies in a Hungarian cohort. Autoimmun Rev. 2014;13(12):1211–9. https://doi.org/10.1016/j.autrev.2014.08.011.
Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken). 2013;65(8):1307–15. https://doi.org/10.1002/acr.21992.
Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65(1):25–34. https://doi.org/10.1016/j.jaad.2010.09.016.
Chen Z, Wang Y, Kuwana M, Xu X, Hu W, Feng X, et al. HLA-DRB1 alleles as genetic risk factors for the development of anti-MDA5 antibodies in patients with dermatomyositis. J Rheumatol. 2017;44(9):1389–93. https://doi.org/10.3899/jrheum.170165.
Lin JM, Zhang YB, Peng QL, Yang HB, Shi JL, Gu ML, et al. Genetic association of HLA-DRB1 multiple polymorphisms with dermatomyositis in Chinese population. HLA. 2017;90(6):354–9. https://doi.org/10.1111/tan.13171.
Tansley SL, Betteridge ZE, Gunawardena H, Jacques TS, Owens CM, Pilkington C, et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther. 2014;16(4):R138. https://doi.org/10.1186/ar4600.
Allenbach Y, Leroux G, Suarez-Calvet X, Preusse C, Gallardo E, Hervier B, et al. Dermatomyositis with or without anti-melanoma differentiation-associated gene 5 antibodies: common interferon signature but distinct NOS2 expression. Am J Pathol. 2016;186(3):691–700. https://doi.org/10.1016/j.ajpath.2015.11.010.
Peng Y, Zhang S, Zhao Y, Liu Y, Yan B. Neutrophil extracellular traps may contribute to interstitial lung disease associated with anti-MDA5 autoantibody positive dermatomyositis. Clin Rheumatol. 2017;37:107–15. https://doi.org/10.1007/s10067-017-3799-y.
Marie I, Hachulla E, Cherin P, Dominique S, Hatron PY, Hellot MF, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum. 2002;47(6):614–22. https://doi.org/10.1002/art.10794.
Schnabel A, Reuter M, Biederer J, Richter C, Gross WL. Interstitial lung disease in polymyositis and dermatomyositis: clinical course and response to treatment. Semin Arthritis Rheum. 2003;32(5):273–84. https://doi.org/10.1053/sarh.2002.50012.
Hosono Y, Nakashima R, Serada S, Murakami K, Imura Y, Yoshifuji H, et al. Splicing factor proline/glutamine-rich is a novel autoantigen of dermatomyositis and associated with anti-melanoma differentiation-associated gene 5 antibody. J Autoimmun. 2017;77:116–22. https://doi.org/10.1016/j.jaut.2016.11.006.
• Matsushita T, Mizumaki K, Kano M, Yagi N, Tennichi M, Takeuchi A, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176(2):395–402. https://doi.org/10.1111/bjd.14882. Novel finding suggesting that an increase in anti-MDA-5 titers is associated with relapse of interstitial lung disease.
Abe Y, Matsushita M, Tada K, Yamaji K, Takasaki Y, Tamura N. Clinical characteristics and change in the antibody titres of patients with anti-MDA5 antibody-positive inflammatory myositis. Rheumatology (Oxford). 2017;56(9):1492–7. https://doi.org/10.1093/rheumatology/kex188.
Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. 2013;23(3):496–502. https://doi.org/10.1007/s10165-012-0663-4.
Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford). 2012;51(9):1563–70. https://doi.org/10.1093/rheumatology/kes102.
Muro Y, Sugiura K, Akiyama M. Limitations of a single-point evaluation of anti-MDA5 antibody, ferritin, and IL-18 in predicting the prognosis of interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Clin Rheumatol. 2013;32(3):395–8. https://doi.org/10.1007/s10067-012-2142-x.
Lee LW, Narang NS, Postolova A, Seminara N, Kantor MA. Anti-MDA5-positive dermatomyositis presenting as fever of unknown origin. J Gen Intern Med. 2016;31(12):1530–6. https://doi.org/10.1007/s11606-016-3769-0.
Chaisson NF, Paik J, Orbai AM, Casciola-Rosen L, Fiorentino D, Danoff S, et al. A novel dermato-pulmonary syndrome associated with MDA-5 antibodies: report of 2 cases and review of the literature. Medicine (Baltimore). 2012;91(4):220–8. https://doi.org/10.1097/MD.0b013e3182606f0b.
Labrador-Horrillo M, Martinez MA, Selva-O'Callaghan A, Trallero-Araguas E, Balada E, Vilardell-Tarres M, et al. Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. J Immunol Res. 2014;2014:290797. https://doi.org/10.1155/2014/290797.
Li L, Wang Q, Wen X, Liu C, Wu C, Yang F, et al. Assessment of anti-MDA5 antibody as a diagnostic biomarker in patients with dermatomyositis-associated interstitial lung disease or rapidly progressive interstitial lung disease. Oncotarget. 2017;8(44):76129–40. https://doi.org/10.18632/oncotarget.19050.
Kawasumi H, Gono T, Kawaguchi Y, Yamanaka H. Recent treatment of interstitial lung disease with idiopathic inflammatory myopathies. Clin Med Insights Circ Respir Pulm Med. 2015;9(Suppl 1):9–17. https://doi.org/10.4137/CCRPM.S23313.
Zou J, Guo Q, Chi J, Wu H, Bao C. HRCT score and serum ferritin level are factors associated to the 1-year mortality of acute interstitial lung disease in clinically amyopathic dermatomyositis patients. Clin Rheumatol. 2015;34(4):707–14. https://doi.org/10.1007/s10067-015-2866-5.
Kotani T, Takeuchi T, Makino S, Hata K, Yoshida S, Nagai K, et al. Combination with corticosteroids and cyclosporin-A improves pulmonary function test results and chest HRCT findings in dermatomyositis patients with acute/subacute interstitial pneumonia. Clin Rheumatol. 2011;30(8):1021–8. https://doi.org/10.1007/s10067-011-1713-6.
Cabezas-Rodriguez I, Morante-Bolado I, Brandy-Garcia A, Queiro-Silva R, Mozo L, Ballina-Garcia FJ. Anti-MDA5 dermatomyositis mimicking psoriatic arthritis. Reumatol Clin. 2016; https://doi.org/10.1016/j.reuma.2016.10.010.
Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford). 2010;49(9):1713–9. https://doi.org/10.1093/rheumatology/keq149.
Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken). 2013;65(8):1316–24. https://doi.org/10.1002/acr.21985.
Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, Lucero MC, Garcia-Herrera A, Espinosa G, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28(8):1097–102. https://doi.org/10.1111/jdv.12300.
Lepelletier C, Bengoufa D, Lyes Z, de Masson A, Chasset F, Jachiet M, et al. Dermatopulmonary syndrome associated with anti-MDA5 antibodies after allogeneic hematopoietic stem cell transplantation. JAMA Dermatol. 2017;2016 https://doi.org/10.1001/jamadermatol.2016.3976.
Avouac J, Guerini H, Wipff J, Assous N, Chevrot A, Kahan A, et al. Radiological hand involvement in systemic sclerosis. Ann Rheum Dis. 2006;65(8):1088–92. https://doi.org/10.1136/ard.2005.044602.
Avouac J, Mogavero G, Guerini H, Drape JL, Mathieu A, Kahan A, et al. Predictive factors of hand radiographic lesions in systemic sclerosis: a prospective study. Ann Rheum Dis. 2011;70(4):630–3. https://doi.org/10.1136/ard.2010.134304.
Valenzuela A, Chung L, Casciola-Rosen L, Fiorentino D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 2014;150(7):724–9. https://doi.org/10.1001/jamadermatol.2013.10416.
Wolstencroft PW, Chung L, Li S, Casciola-Rosen L, Fiorentino DF. Factors associated with clinical remission of skin disease in dermatomyositis. JAMA Dermatol. 2018;2017 https://doi.org/10.1001/jamadermatol.2017.3758.
Oddis CV, Fertig N, Goel A, Espada G, Gregorian MC, Cocco JAM et al. Clinical and serological characterization of the anti-MJ antibody in childhood myositis. Arthritis Rheum-Us. 1997;40(9):652-.
Takahashi K, Yoshida N, Murakami N, Kawata K, Ishizaki H, Tanaka-Okamoto M, et al. Dynamic regulation of p53 subnuclear localization and senescence by MORC3. Mol Biol Cell. 2007;18(5):1701–9. https://doi.org/10.1091/mbc.E06-08-0747.
Ichimura Y, Matsushita T, Hamaguchi Y, Kaji K, Hasegawa M, Tanino Y, et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis. 2012;71(5):710–3. https://doi.org/10.1136/annrheumdis-2011-200697.
Ishikawa A, Muro Y, Sugiura K, Akiyama M. Development of an ELISA for detection of autoantibodies to nuclear matrix protein 2. Rheumatology (Oxford). 2012;51(7):1181–7. https://doi.org/10.1093/rheumatology/kes033.
Rogers A, Chung L, Li S, Casciola-Rosen L, Fiorentino DF. The cutaneous and systemic findings associated with nuclear matrix protein-2 antibodies in adult dermatomyositis patients. Arthritis Care Res (Hoboken). 2017. doi:https://doi.org/10.1002/acr.23210, 69, 1909, 1914.
Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li SF, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1 gamma. Arthritis Rheum-Us. 2013;65(11):2954–62. https://doi.org/10.1002/art.38093.
• Albayda J, Pinal-Fernandez I, Huang W, Parks C, Paik J, Casciola-Rosen L et al. Dermatomyositis patients with anti-nuclear matrix protein-2 autoantibodies have more edema, more severe muscle disease, and increased malignancy risk. Arthritis Care Res (Hoboken). 2017. doi:https://doi.org/10.1002/acr.23188. First paper to comprehensively describe the unique clinical phenotype of anti-NXP-2-positive DM patients.
Espada G, Maldonado Cocco JA, Fertig N, Oddis CV. Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J Rheumatol. 2009;36(11):2547–51. https://doi.org/10.3899/jrheum.090461.
Tansley SL, Betteridge ZE, Shaddick G, Gunawardena H, Arnold K, Wedderburn LR, et al. Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology (Oxford). 2014;53(12):2204–8. https://doi.org/10.1093/rheumatology/keu259.
Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Franceschini F, Quinzanini M, et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther. 2012;14(2):R97. https://doi.org/10.1186/ar3822.
Gunawardena H, Wedderburn LR, Chinoy H, Betteridge ZE, North J, Ollier WE, et al. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum. 2009;60(6):1807–14. https://doi.org/10.1002/art.24547.
Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y, Komura K, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology (Oxford). 2007;46(1):25–8. https://doi.org/10.1093/rheumatology/kel161.
Agricola E, Randall RA, Gaarenstroom T, Dupont S, Hill CS. Recruitment of TIF1gamma to chromatin via its PHD finger-bromodomain activates its ubiquitin ligase and transcriptional repressor activities. Mol Cell. 2011;43(1):85–96. https://doi.org/10.1016/j.molcel.2011.05.020.
Kulkarni A, Oza J, Yao M, Sohail H, Ginjala V, Tomas-Loba A, et al. Tripartite motif-containing 33 (TRIM33) protein functions in the poly(ADP-ribose) polymerase (PARP)-dependent DNA damage response through interaction with amplified in liver cancer 1 (ALC1) protein. J Biol Chem. 2013;288(45):32357–69. https://doi.org/10.1074/jbc.M113.459164.
Fiorentino DF, Kuo K, Chung L, Zaba L, Li S, Casciola-Rosen L. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1gamma antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015;72(3):449–55. https://doi.org/10.1016/j.jaad.2014.12.009.
Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013;65(11):2954–62. https://doi.org/10.1002/art.38093.
Fujimoto M, Murakami A, Kurei S, Okiyama N, Kawakami A, Mishima M, et al. Enzyme-linked immunosorbent assays for detection of anti-transcriptional intermediary factor-1 gamma and anti-Mi-2 autoantibodies in dermatomyositis. J Dermatol Sci. 2016;84(3):272–81. https://doi.org/10.1016/j.jdermsci.2016.09.013.
Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O'Hanlon TP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54(11):3682–9. https://doi.org/10.1002/art.22164.
•• Pinal-Fernandez I, Ferrer-Fabregas B, Trallero-Araguas E, Balada E, Martinez MA, Milisenda JC et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatology (Oxford). 2017. doi:https://doi.org/10.1093/rheumatology/kex413. First study to investigate genetic changes in the TIF1 genes of tumors from patients with paraneoplastic anti-TIF-1γ autoantibody-positive DM.
Fujimoto M, Watanabe R, Ishitsuka Y, Okiyama N. Recent advances in dermatomyositis-specific autoantibodies. Curr Opin Rheumatol. 2016;28(6):636–44. https://doi.org/10.1097/BOR.0000000000000329.
• Mohassel P, Rosen P, Casciola-Rosen L, Pak K, Mammen AL. Expression of the dermatomyositis autoantigen transcription intermediary factor 1gamma in regenerating muscle. Arthritis Rheumatol. 2015;67(1):266–72. https://doi.org/10.1002/art.38863. Illustrates a role for TIF-1γ in muscle regeneration and demonstrates that high expression of TIF-1γ within the muscle may be driving the myositis autoimmune response within DM.
Scholtissek B, Ferring-Schmitt S, Maier J, Wenzel J. Expression of the autoantigen TRIM33/TIF1gamma in skin and muscle of patients with dermatomyositis is upregulated, together with markers of cellular stress. Clin Exp Dermatol. 2017;42(6):659–62. https://doi.org/10.1111/ced.13180.
Bernet LL, Lewis MA, Rieger KE, Casciola-Rosen L, Fiorentino DF. Ovoid palatal patch in dermatomyositis: a novel finding associated with anti-TIF1gamma (p155) antibodies. JAMA Dermatol. 2016;152(9):1049–51. https://doi.org/10.1001/jamadermatol.2016.1429.
Betteridge Z, Gunawardena H, North J, Slinn J, McHugh N. Identification of a novel autoantibody directed against small ubiquitin-like modifier activating enzyme in dermatomyositis. Arthritis Rheum. 2007;56(9):3132–7. https://doi.org/10.1002/art.22862.
Muro Y, Sugiura K, Akiyama M. Low prevalence of anti-small ubiquitin-like modifier activating enzyme antibodies in dermatomyositis patients. Autoimmunity. 2013;46(4):279–84. https://doi.org/10.3109/08916934.2012.755958.
Fujimoto M, Matsushita T, Hamaguchi Y, Kaji K, Asano Y, Ogawa F, et al. Autoantibodies to small ubiquitin-like modifier activating enzymes in Japanese patients with dermatomyositis: comparison with a UK Caucasian cohort. Ann Rheum Dis. 2013;72(1):151–3. https://doi.org/10.1136/annrheumdis-2012-201736.
Ge Y, Lu X, Shu X, Peng Q, Wang G. Clinical characteristics of anti-SAE antibodies in Chinese patients with dermatomyositis in comparison with different patient cohorts. Sci Rep. 2017;7(1):188. https://doi.org/10.1038/s41598-017-00240-6.
Betteridge ZE, Gunawardena H, Chinoy H, North J, Ollier WE, Cooper RG, et al. Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann Rheum Dis. 2009;68(10):1621–5. https://doi.org/10.1136/ard.2008.097162.
Tarricone E, Ghirardello A, Rampudda M, Bassi N, Punzi L, Doria A. Anti-SAE antibodies in autoimmune myositis: identification by unlabelled protein immunoprecipitation in an Italian patient cohort. J Immunol Methods. 2012;384(1–2):128–34. https://doi.org/10.1016/j.jim.2012.07.019.
Lee S, Findeisen J, McLean C, Stavrakoglou A. Recalcitrant ulcers associated with anti-small ubiquitin-like modifier activating enzyme-positive dermatomyositis treated with surgery followed by intravenous immunoglobulin. Australas J Dermatol. 2017;59:e76–8. https://doi.org/10.1111/ajd.12659.
Muro Y, Sugiura K, Nara M, Sakamoto I, Suzuki N, Akiyama M. High incidence of cancer in anti-small ubiquitin-like modifier activating enzyme antibody-positive dermatomyositis. Rheumatology (Oxford). 2015;54(9):1745–7. https://doi.org/10.1093/rheumatology/kev247.
Lega JC, Fabien N, Reynaud Q, Durieu I, Durupt S, Dutertre M, et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun Rev. 2014;13(9):883–91. https://doi.org/10.1016/j.autrev.2014.03.004.
Kalluri M, Sahn SA, Oddis CV, Gharib SL, Christopher-Stine L, Danoff SK, et al. Clinical profile of anti-PL-12 autoantibody. Cohort study and review of the literature. Chest. 2009;135(6):1550–6. https://doi.org/10.1378/chest.08-2233.
Ang CC, Anyanwu CO, Robinson E, Okawa J, Feng R, Fujimoto M, et al. Clinical signs associated with an increased risk of interstitial lung disease: a retrospective study of 101 patients with dermatomyositis. Br J Dermatol. 2017;176(1):231–3. https://doi.org/10.1111/bjd.14801.
Labirua-Iturburu A, Selva-O'Callaghan A, Vincze M, Danko K, Vencovsky J, Fisher B, et al. Anti-PL-7 (anti-threonyl-tRNA synthetase) antisynthetase syndrome: clinical manifestations in a series of patients from a European multicenter study (EUMYONET) and review of the literature. Medicine (Baltimore). 2012;91(4):206–11. https://doi.org/10.1097/MD.0b013e318260977c.
Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One. 2013;8(4):e60442. https://doi.org/10.1371/journal.pone.0060442.
Johnson C, Connors GR, Oaks J, Han S, Truong A, Richardson B, et al. Clinical and pathologic differences in interstitial lung disease based on antisynthetase antibody type. Respir Med. 2014;108(10):1542–8. https://doi.org/10.1016/j.rmed.2014.09.003.
• Mescam-Mancini L, Allenbach Y, Hervier B, Devilliers H, Mariampillay K, Dubourg O, et al. Anti-Jo-1 antibody-positive patients show a characteristic necrotizing perifascicular myositis. Brain. 2015;138(Pt 9):2485–92. https://doi.org/10.1093/brain/awv192. Results indicate that perifascicular necrosis is a unique phenotype of anti-Jo-1-positive DM.
Bartoloni E, Gonzalez-Gay MA, Scire C, Castaneda S, Gerli R, Lopez-Longo FJ, et al. Clinical follow-up predictors of disease pattern change in anti-Jo1 positive anti-synthetase syndrome: results from a multicenter, international and retrospective study. Autoimmun Rev. 2017;16(3):253–7. https://doi.org/10.1016/j.autrev.2017.01.008.
Cox JT, Gullotti DM, Mecoli CA, Lahouti AH, Albayda J, Paik J, et al. “Hiker’s feet”: a novel cutaneous finding in the inflammatory myopathies. Clin Rheumatol. 2017;36(7):1683–6. https://doi.org/10.1007/s10067-017-3598-5.
Bachmeyer C, Tillie-Leblond I, Lacert A, Cadranel J, Aractingi S. “Mechanic’s hands”: a misleading cutaneous sign of the antisynthetase syndrome. Br J Dermatol. 2007;156(1):192–4. https://doi.org/10.1111/j.1365-2133.2006.07593.x.
Hozumi H, Enomoto N, Kono M, Fujisawa T, Inui N, Nakamura Y, et al. Prognostic significance of anti-aminoacyl-tRNA synthetase antibodies in polymyositis/dermatomyositis-associated interstitial lung disease: a retrospective case control study. PLoS One. 2015;10(3):e0120313. https://doi.org/10.1371/journal.pone.0120313.
Hozumi H, Fujisawa T, Nakashima R, Johkoh T, Sumikawa H, Murakami A, et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med. 2016;121:91–9. https://doi.org/10.1016/j.rmed.2016.10.019.
Rosen A, Casciola-Rosen L. Autoantigens as partners in initiation and propagation of autoimmune rheumatic diseases. Annu Rev Immunol. 2016;34:395–420. https://doi.org/10.1146/annurev-immunol-032414-112205.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Inflammatory Muscle Disease
Rights and permissions
About this article
Cite this article
Wolstencroft, P.W., Fiorentino, D.F. Dermatomyositis Clinical and Pathological Phenotypes Associated with Myositis-Specific Autoantibodies. Curr Rheumatol Rep 20, 28 (2018). https://doi.org/10.1007/s11926-018-0733-5
Published:
DOI: https://doi.org/10.1007/s11926-018-0733-5