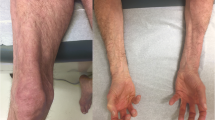
Abstract
The idiopathic inflammatory myopathies (IIM) are a group of autoimmune diseases traditionally defined by clinical manifestations including skeletal muscle weakness, skin rashes, elevated skeletal muscle enzymes, and neurophysiological and/or histological evidence of muscle inflammation. Patients with myositis overlap can develop other features including parenchymal lung disease, inflammatory arthritis, gastrointestinal manifestations and marked constitutional symptoms. Although patients may be diagnosed as having polymyositis (PM) or dermatomyositis (DM) under the IIM spectrum, it is quite clear that disease course between subgroups of patients is different. For example, interstitial lung disease may predominate in some, whereas cutaneous complications, cancer risk, or severe refractory myopathy may be a significant feature in others. Therefore, tools that facilitate diagnosis and indicate which patients require more detailed investigation for disease complications are invaluable in clinical practice. The expanding field of autoantibodies (autoAbs) associated with connective tissue disease (CTD)-myositis overlap has generated considerable interest over the last few years. Using an immunological diagnostic approach, this group of heterogeneous conditions can be separated into a number of distinct clinical phenotypes. Rather than diagnose a patient as simply having PM, DM or overlap CTD, we can define syndromes to differentiate disease subsets that emphasise clinical outcomes and guide management. There are now over 15 CTD-myositis overlap autoAbs found in patients with a range of clinical manifestations including interstitial pneumonia, cutaneous disease, cancer-associated myositis and autoimmune-mediated necrotising myopathy. This review describes their diagnostic utility, potential role in disease monitoring and response to treatment. In the future, routine use of these autoAb will allow a stratified approach to managing this complex set of conditions.

Similar content being viewed by others
References
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292(7):344–347
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292(8):403–407
Tanimoto K, Nakano K, Kano S, Mori S, Ueki H, Nishitani H, Sato T, Kiuchi T, Ohashi Y (1995) Classification criteria for polymyositis and dermatomyositis. J Rheumatol 22(4):668–674
Gunawardena H, Betteridge ZE, McHugh NJ (2009) Myositis specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology 48(6):607–612
Tansley S, Gunawardena H (2014) The evolving spectrum of polymyositis and dermatomyositis--moving towards clinicoserological syndromes: a critical review. Clin Rev Allergy Immunol 47(3):264–273
Kang EH, Kuwana M, Okazaki Y, Lee EY, Lee YJ, Lee EB et al (2014) Comparison of radioimmunoprecipitation versus antigen-specific assays for identification of myositis-specific autoantibodies in dermatomyositis patients. Mod Rheumatol 24(6):945–948
Ronnelid J, Barbasso Helmers S, Storfors H, Grip K, Ronnblom L, Franck-Larsson K et al (2009) Use of a commercial line blot assay as a screening test for autoantibodies in inflammatory myopathies. Autoimmun Rev 9(1):58–61
Nakashima R, Imura Y, Hosono Y, Seto M, Murakami A, Watanabe K et al (2014) The multicenter study of a New assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS ONE 9(1), e85062
Aggarwal R, Oddis CV, Goudeau D, Fertig N, Metes I, Stephens C et al. (2014) Anti-signal recognition particle autoantibody ELISA validation and clinical associations. Rheumatology Dec 17 pii: keu436 [Epub ahead of print]
Hane H, Muro Y, Watanabe K, Ogawa Y, Sugiura K, Akiyama M (2014) Establishment of an ELISA to detect anti-glycyl-tRNA synthetase antibody (anti-EJ), a serological marker of dermatomyositis/polymyositis and interstitial lung disease. Clin Chim Acta 431:9–14
Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, Kuwana M (2009) RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 60(7):2193–2200
Aggarwal R, Oddis CV, Goudeau D, Fertig N, Metes I, Stephens C et al (2014) Anti-transcription intermediary factor 1-gamma autoantibody ELISA development and validation. Rheumatology 53(3):433–437
Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A et al (2012) Anti-TIF1 antibodies (antip155) in adult patients with dermatomyositis: comparison of different diagnostic assays. Ann Rheum Dis 71(6):993–996
Ishikawa A, Muro Y, Sugiura K, Akiyama M (2012) Development of an ELISA for detection of autoantibodies to nuclear matrix protein 2. Rheumatology 51(7):1181–1187
Muro Y, Sugiura K, Akiyama M (2013) A new ELISA for dermatomyositis autoantibodies: rapid introduction of autoantigen cDNA to recombinant assays for autoantibody measurement. Clin Dev Immunol 2013:856815
Mammen AL, Chung T, Christopher-Stine L (2011) Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin associated autoimmune myopathy. Arthritis Rheum 63(3):713–721
Musset L, Miyara M, Benveniste O, Charuel J-L, Shikhman A, Boyer O et al (2014) Analysis of Autoantibodies to 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase Using Different Technologies. Int J Immunol Res 2014:405956
Drouot L, Allenbach Y, Jouen F, Charuel J-L, Martinet JRM, Meyer A et al (2014) Exploring necrotizing autoimmune myopathies with a novel immunoassay for anti-3-hydroxy-3- methyl-glutaryl-CoA reductase autoantibodies. Arthritis Res Ther 16(1):1–11
Friedman AW, Targoff IN, Arnett FC (1996) Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 26(1):459–467
Kalluri M, Sahn SA, Oddis CV, Gharib SL, Christopher-Stine L, Danoff SK et al (2009) Clinical profile of anti-PL-12 autoantibody: cohort study and review of the literature. Chest 135(6):1550–1556
Steen VD (2005) Autoantibodies in systemic sclerosis. Semin Arthritis Rheum 35(1):35–42
Debray MP, Borie R, Revel MP, Naccache JM, Khalil A, Toper C et al (2015) Interstitial lung disease in anti-synthetase syndrome: initial and follow up CT findings. Eur J Radiol 84(3):516–523
Fischer A, Brown KK (2015) Interstitial lung disease in undifferentiated forms of connective tissue disease. Arthritis Care Res 67(1):4–11
Marguerie C, Bunn CC, Beynon HL, Bernstein RM, Hughes JM, So AK, Walport MJ (1990) Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med 77(282):1019–1038
Hallowell RW, Ascherman DP, Danoff SK (2014) Pulmonary manifestations of polymyositis/dermatomyositis. Semin Respir Crit Care Med 35(2):239–248
Hallowell RW, Danoff SK (2014) Interstitial lung disease associated with the idiopathic inflammatory myopathies and the antisynthetase syndrome: recent advances. Curr Opin Rheumatol 26(6):684–689
Watanabe K, Handa T, Tanizawa K, Hosono Y, Taguchi Y, Noma S et al (2011) Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med 105(8):1238–1472
Schneider F, Yousem SA, Bi D, Gibson KF, Oddis CV, Aggarwal R (2014) Pulmonary pathologic manifestations of anti-glycl-tRNA synthetase (anti-EJ)- related inflammatory myopathy. J Clin Pathol 67(8):678–683
Marie I, Josse S, Decaux O, Diot E, Landron C, Roblot P et al (2013) Clinical manifestations and outcome of anti-PL7 positive patients with anti-synthetase syndrome. Eur J Intern Med 24(5):474–479
Johnson C, Connors GR, Oaks J, Han S, Truong A, Richardson B et al (2014) Clinical and pathologic differences in interstitial lung disease based on antisynthetase antibody type. Respir Med 108(10):1542–1548
Marie I, Josse S, Decaux O, Dominique S, Landron C, Roblot P et al (2013) Outcome of anti-PL12 positive patients with antisynthetase syndrome. Presse Med 42(6):e153–e158
Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C et al (2012) Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 11(10):739–745
Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M et al (2013) Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 8(4), e60442
Lefevre G, Meyer A, Launay D, Machelart I, DeBandt M, Michaud J et al (2014) Seronegative polyarthritis revealing antisynthetase syndrome: a multicentre study of 40 patients. Rheumatology 27
Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T et al (2005) Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52(5):1571–1576
Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L (2011) The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 65(1):25–34
Tansley SL, Betteridge ZE, Gunawardena H, Jacques TS, Owens CM, Pilkington C et al (2014) Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther 16(4):R138
Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Tincani A, Selmi C et al (2014) Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin Exp Rheumatol 32(6):891–897
Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, Trallero-Araguas E, Balada E, Vilardell-Tarres M, Juárez C (2014) Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. Int J Immunol Res 2014:290797
Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K et al (2010) Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology 49(9):1713–1719
Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y et al (2012) Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology 51(9):1563–1567
Chaisson NF, Paik J, Orbai AM, Casciola-Rosen L, Fiorentino D, Danoff S, Rosen A (2012) A novel dermato-pulmonary syndrome associated with MDA-5 antibodies: report of 2 cases and review of the literature. Medicine (Baltimore) 91(4):220–228
Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, Christopher-Stine L (2013) Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res 65(8):1307–1315
Kobayashi I, Okura Y, Yamada M, Kawamura N, Kuwana M, Ariga T (2011) Anti-melanoma differentiation -associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J Pediatr 158(4):675–677
Kobayashi N, Takezaki S, Kobayashi I, Iwata N, Mori M, Nagai K et al (2014) Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology
Aggarwal R, Lucas M, Fertig N, Oddis CV, Medsger TA Jr (2009) Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum 60(4):1112–1118
Koschik RW 2nd, Fertig N, Lucas MR, Domsic RT, Medsger TA Jr (2012) Anti-PM-Scl antibody in patients with systemic sclerosis. Clin Exp Rheumatol 30(2):S12–S16
D’Aoust J, Hudson M, Tatibouet S, Wick J, Canadian Scleroderma Research Group, Mahler M, Baron M, Fritzler MJ (2014) Clinical and serologic correlates of anti-PM/Scl antibodies in systemic sclerosis: a multicenter study of 763 patients. Arthritis Rheum 66(6):1608–1615
Lega JC, Cottin V, Fabien N, Thivolet-Béjui F, Cordier JF (2010) Interstitial lung disease associated with anti-PM/Scl or anti-aminoacyl-tRNA synthetase autoantibodies: a similar condition? J Rheumatol 37(5):1000–1009
Hanke K, Brückner CS, Dähnrich C, Huscher D, Komorowski L, Meyer W et al (2009) Antibodies against PM/Scl-75 and PM/Scl-100 are independent markers for different subsets of systemic sclerosis patients. Arthritis Res Ther 11(1):R22
Guillen-Del Castillo A, Pilar Simeón-Aznar C, Fonollosa-Pla V, Alonso-Vila S, Reverte-Vinaixa MM, Muñoz X et al (2014) Good outcome of interstitial lung disease in patients with scleroderma associated to anti-PM/Scl antibody. Semin Arthritis Rheum 44(3):331–337
Arnett FC, Reveille JD, Goldstein R, Pollard KM, Leaird K, Smith EA, Leroy EC, Fritzler MJ (1996) Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum 39(7):1151–1160
Tormey VJ, Bunn CC, Denton CP, Black CM (2001) Anti-fibrillarin antibodies in systemic sclerosis. Rheumatology 40(10):1157–1162
Kaji K, Fertig N, Medsger TA Jr, Satoh T, Hoshino K, Hamaguchi Y et al (2014) Autoantibodies to RuvBL1 and RuvBL2: a novel systemic sclerosis-related antibody associated with diffuse cutaneous and skeletal muscle involvement. Arthritis Care Res 66(4):575–584
Olazagasti JM, Baez PJ, Wetter DA, Ernste FC (2015) Cancer risk in dermatomyositis: a meta-analysis of cohort studies. Am J Clin Dermatol 16(2):89–98
Chinoy H, Fertig N, Oddis CV, Ollier WE, Cooper RG (2007) The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis 66(10):1345–1349
Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O’Hanlon TP, Miller FW, Rider LG, Childhood Myositis Heterogeneity Study Group; International Myositis Collaborative Study Group (2006) A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum 54(11):3682–3689
Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y et al (2007) Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology 46(1):25–28
Trallero-Araguás E, Labrador-Horrillo M, Selva-O’Callaghan A, Martínez MA, Martínez-Gómez X, Palou E, Rodriguez-Sanchez JL, Vilardell-Tarrés M (2010) Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine (Baltimore) 89(1):47–52
Fujimoto M, Hamaguchi Y, Kaji K, Matsushita T, Ichimura Y, Kodera M et al (2012) Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum 64(2):513–522
Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O’Callaghan A, Martínez-Gómez X, Bosch X, Labrador-Horrillo M, Grau-Junyent JM, Vilardell-Tarrés M (2012) Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum 64(2):523–532
Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M (2010) Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 22(6):627–632
Fiorentino DF, Kuo K, Chung L, Zaba L, Li S, Casciola-Rosen L (2015) Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J Am Acad Dermatol 72(3):449–455
Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, Rosen A, Casciola-Rosen L (2013) Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum 65(11):2954–2962
Ichimura Y, Matsushita T, Hamaguchi Y, Kaji K, Hasegawa M, Tanino Y et al (2012) Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis 71(5):710–713
Gunawardena H, Wedderburn LR, North J, Betteridge Z, Dunphy J, Chinoy H et al (2008) Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology 47(3):324–328
Bingham A, Mamyrova G, Rother KI, Oral E, Cochran E, Premkumar A et al (2008) Predictors of acquired lipodystrophy in juvenile-onset dermatomyositis and a gradient of severity. Medicine (Baltimore) 87(2):70–86
Espada G, Maldonado Cocco JA, Fertig N, Oddis CV (2009) Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J Rheumatol 36(11):2547–2551
Gunawardena H, Wedderburn LR, Chinoy H, Betteridge ZE, North J, Ollier WE et al (2009) Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum 60(6):1807–1814
Tansley SL, Betteridge ZE, Shaddick G, Gunawardena H, Arnold K, Wedderburn LR, McHugh NJ, Juvenile Dermatomyositis Research Group (2014) Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology 53(12):2204–2208
Valenzuela A, Chung L, Casciola-Rosen L, Fiorentino D (2014) Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol 150(7):724–729
Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Franceschini F, Quinzanini M et al (2012) Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther 14(2):R97
Sugiura K, Muro Y, Akiyama M (2012) Autoantibodies to nuclear matrix protein 2/MJ in adult-onset dermatomyositis with severe calcinosis. J Am Acad Dermatol 67(4):e167–e168
Shah AA, Casciola-Rosen L, Rosen A (2015) Review: cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheum 67(2):317–326
Betteridge ZE, Gunawardena H, Chinoy H, North J, Ollier WE, Cooper RG, McHugh NJ, UK Adult Onset Myositis Immunogenetic Collaboration (2009) Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann Rheum Dis 68(10):1621–1625
Tarricone E, Ghirardello A, Rampudda M, Bassi N, Punzi L, Doria A (2012) Anti-SAE antibodies in autoimmune myositis: identification by unlabelled protein immunoprecipitation in an Italian patient cohort. J Immunol Methods 384(1–2):128–134
Muro Y, Sugiura K, Akiyama M (2013) Low prevalence of anti-small ubiquitin-like modifier activating enzyme antibodies in dermatomyositis patients. Autoimmunity 46(4):279–284
Bodoki L, Nagy-Vincze M, Griger Z, Betteridge Z, Szöllősi L, Dankó K (2014) Four dermatomyositis-specific autoantibodies-anti-TIF1γ, anti-NXP2, anti-SAE and anti-MDA5-in adult and juvenile patients with idiopathic inflammatory myopathies in a Hungarian cohort. Autoimmun Rev 13(12):1211–1219
Fujimoto M, Matsushita T, Hamaguchi Y, Kaji K, Asano Y, Ogawa F et al (2013) Autoantibodies to small ubiquitin-like modifier activating enzymes in Japanese patients with dermatomyositis: comparison with a UK Caucasian cohort. Ann Rheum Dis 72(1):151–153
Targoff IN, Reichlin M (1985) The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum 28(7):796–803
Okada S, Weatherhead E, Targoff IN, Wesley R, Miller FW, International Myositis Collaborative Study Group (2003) Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum 48(8):2285–2293
Love LA, Weinberg CR, McConnaughey DR, Oddis CV, Medsger TA Jr, Reveille JD, Arnett FC, Targoff IN, Miller FW (2009) Ultraviolet radiation intensity predicts the relative distribution of dermatomyositis and anti-Mi-2 autoantibodies in women. Arthritis Rheum 60(8):2499–2504
Petri MH, Satoh M, Martin-Marquez BT, Vargas-Ramírez R, Jara LJ, Saavedra MA et al (2013) Implications in the difference of anti-Mi-2 and -p155/140 autoantibody prevalence in two dermatomyositis cohorts from Mexico City and Guadalajara. Arthritis Res Ther 4;15(2):R48
Rider LG, Shah M, Mamyrova G, Huber AM, Rice MM, Targoff IN, Miller FW, Childhood Myositis Heterogeneity Collaborative Study Group (2013) The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 92(4):223–243
Brown DA, Simoni RD (1984) Biogenesis of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an integral glycoprotein of the endoplasmic reticulum. Proc Natl Acad Sci U S A 81(6):1674–1678
Olender EH, Simon RD (1992) The intracellular targeting and membrane topology of 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem 25;267(6):4223–4235
Hengstman GJ, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J et al (2006) Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis 65(12):1635–1638
Targoff IN, Johnson AE, Miller FW (1990) Antibody to signal recognition particle in polymyositis (1990). Arthritis Rheum 33(9):1361–1370
Hengstman GJ, Brouwer R, Egberts WT, Seelig HP, Jongen PJ, van Venrooij WJ, van Engelen BG (2002) Clinical and serological characteristics of 125 Dutch myositis patients. Myositis specific autoantibodies aid in the differential diagnosis of the idiopathic inflammatory myopathies. J Neurol 249(1):69–75
Basnayake SK, Blumbergs P, Tan JA, Roberts-Thompson PJ, Limaye V (2015) Inflammatory myopathy with anti-SRP antibodies: case series of a South Australian cohort. Clin Rheumatol 34(3):603–608
Miller T, Al-Lozi MT, Lopate G, Pestronk A (2002) Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry 73(4):420–428
Suzuki S, Hayashi YK, KuwanaM TR, Suzuki N, Nishino I (2012) Myopathy associated with antibodies to signal recognition particle: disease progression and neurological outcome. Arch Neurol 69(6):728–732
Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C, American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute (2002) ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 40(3):567–572
Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL (2010) A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 62(9):2757–2766
Mammen AL, Gaudet D, Brisson D, Christopher-Stine L, Lloyd TE, Leffell MS, Zachary AA (2012) Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res 64(8):1233–1237
Limaye V, Bundell C, Hollingsworth P, Rojana-Udomsart A, Mastaglia F, Blumbergs P, Lester S (2014) Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve. doi:10.1002/mus.24541
SEARCH Collaborative Group, Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R (2008) SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med 21;359(8):789–799
Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA (2010) Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 41(2):185–190
Werner JL, Christopher-Stine L, Ghazarian SR, Pak KS, Kus JE, Daya NR, Lloyd TE, Mammen AL (2012) Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 64(12):4087–4093
Stone KB, Oddis CV, Fertig N, Katsumata Y, Lucas M, Vogt M, Domsic R, Ascherman DP (2007) Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum 56(9):3125–3131
Sato S, Kuwana M, Fujita T, Suzuki Y (2013) Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol 23(3):496–502
Muro Y, Sugiura K, Hoshino K, Akiyama M (2012) Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology 51:800–804
Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP, Oddis CV (2014) Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 73(1):227–232
Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T et al (2010) The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 49(3):433–440
Gordon PA, Winer JB, Hoogendijk JE, Choy EH (2012) Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 15(8):CD003643
Yamasaki Y, Yamada H, Yamasaki M, Ohkubo M, Azuma K, Matsuoka S et al (2007) Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology 46(1):124–130
Morganroth PA, Kreider ME, Werth VP (2010) Mycophenolate mofetil for interstitial lung disease in dermatomyositis. Arthritis Care Res 62(10):1496–1501
Fischer A, Brown KK, Du Bois RM, Frankel SK, Cosgrove GP, Fernandez-Perez ER et al (2013) Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol 40(5):640–646
Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV (2005) Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 52(8):2439–2446
Labirua-Iturburu A, Selva-O’Callaghan A, Martínez-Gómez X, Trallero-Araguás E, Labrador-Horrillo M, Vilardell-Tarrés M (2013) Calcineurin inhibitors in a cohort of patients with antisynthetase-associated interstitial lung disease. Clin Exp Rheumatol 31(3):436–439
Cavagna L, Caporali R, Abdì-Alì L, Dore R, Meloni F, Montecucco C (2013) Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. J Rheumatol 40(4):484–492
Kurita T, Yasuda S, Amengual O, Atsumi T (2015) The efficacy of calcineurin inhibitors for the treatment of interstitial lung disease associated with polymyositis/dermatomyositis. Lupus 24(1):3–9
Muro Y, Sugiura K, Akiyama M (2013) Limitations of a single-point evaluation of anti-MDA5 antibody, ferritin, and IL-18 in predicting the prognosis of interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Clin Rheumatol 32(3):395–398
Tanaka F, Origuchi T, Migita K, Tominaga M, Kawakami A, Kawabe Y, Eguchi K (2000) Successful combined therapy of cyclophosphamide and cyclosporine for acute exacerbated interstitial pneumonia associated with dermatomyositis. Intern Med 39(5):428–430
Kameda H, Nagasawa H, Ogawa H, Sekiguchi N, Takei H, Tokuhira M, Amano K, Takeuchi T (2005) Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol 32(9):1719–1726
Levine TD (2005) Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum 52(2):601–607
Valiyil R, Casciola-Rosen L, Hong G, Mammen A, Christopher-Stine L (2010) Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res 62(9):1328–1334
Couderc M, Gottenberg JE, Mariette X, Hachulla E, Sibilia J, Fain O et al (2011) Efficacy and safety of rituximab in the treatment of refractory inflammatory myopathies in adults: results from the AIR registry. Rheumatology 50(12):2283–2289
Bader-Meunier B, Decaluwe H, Barnerias C, Gherardi R, Quartier P, Faye A et al (2011) Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol 38(7):1436–1440
Mahler EA, Blom M, Voermans NC, van Engelen BG, van Riel PL, Vonk MC (2011) Rituximab treatment in patients with refractory inflammatory myopathies. Rheumatology 50(12):2206–2213
Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC et al (2013) Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 65(2):314–324
Aggarwal R, Bandos A, Reed AM, Ascherman DP, Barohn RJ, Feldman BM (2014) Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheum 66(3):740–749
Marie I, Dominique S, Janvresse A, Levesque H, Menard JF (2012) Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 106(4):581–587
Clottu A, Laffitte E, Prins C, Chizzolini C (2012) Response of mucocutaneous lesions to rituximab in a case of melanoma differentiation antigen 5-related dermatomyositis. Dermatology 225(4):376–380
Nalotto L, Iaccarino L, Zen M, Gatto M, Borella E, Domenighetti M, Punzi L, Doria A (2013) Rituximab in refractory idiopathic inflammatory myopathies and antisynthetase syndrome: personal experience and review of the literature. Immunol Res 56(2–3):362–370
Muñoz-Beamud F, Isenberg DA (2014) Rituximab as an effective alternative therapy in refractory idiopathic inflammatory myopathies. Clin Exp Rheumatol 31(6):896–903
Unger L, Kampf S, Lüthke K, Aringer M (2014) Rituximab therapy in patients with refractory dermatomyositis or polymyositis: differential effects in a real-life population. Rheumatology 53(9):1630–1638
Andersson H, Sem M, Lund MB, Aaløkken TM, Günther A, Walle-Hansen R, Garen T (2015) Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology
Koichi Y, Aya Y, Megumi U, Shunichi K, Masafumi S, Hiroaki M et al (2015) A case of anti-MDA5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Mod Rheumatol 12:1–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunawardena, H. The Clinical Features of Myositis-Associated Autoantibodies: a Review. Clinic Rev Allerg Immunol 52, 45–57 (2017). https://doi.org/10.1007/s12016-015-8513-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8513-8