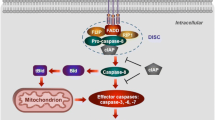
Abstract
Despite the progress that has been made over the past two decades in cardiovascular research, heart failure remains a major cause of morbidity and mortality worldwide. Insight into the cellular and molecular mechanisms that underlie the heart failure in individuals with ischemic heart disease have identified defects in cellular processes that govern autophagy, apoptosis and necrosis as a prevailing underlying cause. Indeed, programmed cell death of cardiac cells by apoptosis or necrosis is believed to involve the intrinsic mitochondrial pathway and/or extrinsic death receptor pathway by certain Bcl-2 family members as well as components of the TNFα signaling pathway. In this review, we discuss recent advances in the molecular signaling factors that govern cardiac cell fate under normal and disease conditions.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–220.
Statistics Canada. Morality, Summary List of Causes 2008. 2011.
Kirshenbaum LA, Schneider MD. Adenovirus E1A represses cardiac gene transcription and reactivates DNA synthesis in ventricular myocytes, via alternative pocket protein- and p300-binding domains. J Biol Chem. 1995;270:7791–4.
Kirshenbaum LA, Abdellatif M, Chakraborty S, Schneider MD. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev Biol. 1996;179(2):402–11.
Kirshenbaum LA, Schneider MD. The cardiac cell cycle, pocket proteins, and p300. Trends Cardiovasc Med. 1995;5(6):230–5.
Leri A, Kajstura J, Anversa P. Mechanisms of myocardial regeneration. Trends Cardiovasc Med. 2011;21(2):52–8.
Regula KM, Kirshenbaum LA. Apoptosis of ventricular myocytes: a means to an end. J Mol Cell Cardiol. 2005;38(1):3–13.
de Moissac D, Zheng H, Kirshenbaum LA. Linkage of the BH4 domain of Bcl-2 and the nuclear factor kappaB signaling pathway for suppression of apoptosis. J Biol Chem. 1999;274(41):29505–9.
Shaw J, Kirshenbaum LA. Molecular regulation of autophagy and apoptosis during ischemic and non-ischemic cardiomyopathy. Autophagy. 2008;4(4):427–34.
Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21(3):485–95.
Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1(2):120–9.
• Singh SS, Kang PM. Mechanisms and inhibitors of apoptosis in cardiovascular diseases. Curr Pharm Des. 2011;17(18):1783–93. Recent review highlighting the apoptotic pathways associated with heart failure.
Goljan E. Rapid review pathology. 3rd ed. Philadelphia: Mosby/Elsevier; 2010.
Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306.
McCall K. Genetic control of necrosis - another type of programmed cell death. Curr Opin Cell Biol. 2010;22:882–8.
Inoue Y, Klionsky DJ. Regulation of macroautophagy in Saccharomyces cerevisiae. Semin Cell Dev Biol. 2010;21(7):664–70.
Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10(7):458–67.
Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8(8):622–32.
Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333(1–2):169–74.
Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;32(7020):1032–6.
Valentim L, Laurence KM, Townsend PA, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40(6):846–52.
Hariharan N, Zhai P, Sadoshima J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal. 2011;14(11):2179–90.
Takemura G, Miyata S, Kawase Y, et al. Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy. 2006;2(3):212–4.
Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;19(Pt 2):259–70.
Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–39.
Zalckvar E, Berissi H, Mizrachy L, et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10(3):285–92.
Criollo A, Niso-Santano M, Malik SA, et al. Inhibition of autophagy by TAB2 and TAB3. EMBO J. 2011;30(24):4908–20.
•• Zhu Y, Zhao L, Liu L, et al. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell. 2010;1(5):468–77. Recent study highlighting the cross talk between autophagy and apoptosis.
Kerr JFR. History of the events leading to the formulation of the apoptosis concept. Toxicology. 2002;181–182:471–4.
Kinchen JM. A model to die for: signaling to apoptotic cell removal in worm, fly and mouse. Apoptosis. 2010;15(9):998–1006.
Crocoll A, Herzer U, Ghyselinck NB, et al. Interdigital apoptosis and downregulation of BAG-1 expression in mouse autopods. Mech Dev. 2002;111(1–2):149–52.
de Moissac D, Gurevich RM, Zheng H, et al. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol. 2000;32:53–63.
Prech M, Marszalek A, Marszalek J, et al. Apoptosis as a mechanism for the elimination of cardiomyocytes after acute myocardial infarction. Am J Cardiol. 2010;105:1240–5.
Weidman D, Shaw J, Bednarczyk J, et al. Dissecting apoptosis and intrinsic death pathways in the heart. Methods Enzymol. 2008;446:277–85.
Du C, Fang M, Li Y, et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42.
Suzuki Y, Imai Y, Nakayama H, et al. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8(3):613–21.
Whelan RS, Konstantinidis K, Wei A-C, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA. 2012;109(17):6566–71.
Zamorano S, Rojas-Rivera D, Lisbona F, et al. A BAX/BAK and Cyclophilin D-Independent Intrinsic Apoptosis Pathway. PLoS One. 2012;7(6):e37782.
Robaye B, Mosselmans R, Fiers W, et al. Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am J Pathol. 1991;38(2):447–53.
Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75(6):1169–78.
Pollard T. Cell biology. 2nd ed. Philadelphia: Saunders/Elsevier; 2008.
Marchetti P, Castedo M, Susin SA, et al. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184(3):1155–60.
Chan FK-M, Shisler J, Bixby JG, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–21.
• Cho YS, Park SY, Shin HS, Chan FK-M. Physiological consequences of programmed necrosis, an alternative form of cell demise. Mol Cells. 2010;29(4):327–32. Recent review highlighting advances in programmed necrosis.
Vande Velde C, Cizeau J, Dubik D, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20(15):5454–68.
Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–21.
•• Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108(8):1017–36. Most recent systematic review about programmed cell death in the myocardium.
Lemasters JJ, Qian T, Bradham CA, et al. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr. 1999;31:305–19.
• Dhingra R, Shaw JA, Aviv Y, Kirshenbaum LA. Dichotomous actions of NF-kappaB signaling pathways in heart. J Cardiovasc Transl Res. 2010;3:344–54. Recent review highlighting cell survival signaling in the heart.
Ea C-K, Deng L, Xia ZP, et al. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–57.
Park S-M, Yoon J-B, Lee TH. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 2004;566:151–6.
Vince JE, Pantaki D, Feltham R, et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis. J Biol Chem. 2009;284:35906–15.
Mahul-Mellier AL, Pazarentzos E, Datler C, et al. De-ubiquitinating protease USP2a targets RIP1 and TRAF2 to mediate cell death by TNF. Cell Death Differ. 2012;19(5):891–9.
Wertz IE, O'Rourke KM, Zhou H, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–9.
Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2011;21:22–39.
Poyet JL, Srinivasula SM, Lin JH, et al. Activation of the Ikappa B kinases by RIP via IKKgamma /NEMO-mediated oligomerization. J Biol Chem. 2000;275:37966–77.
Kim Y-J, Kim H-C, Ko H, et al. Stercurensin inhibits nuclear factor-κB-dependent inflammatory signals through attenuation of TAK1-TAB1 complex formation. J Cell Biochem. 2010. doi:10.1002/jcb.22945.
Regula KM, Baetz D, Kirshenbaum LA. Nuclear factor-kappaB represses hypoxia-induced mitochondrial defects and cell death of ventricular myocytes. Circulation. 2004;110:3795–802.
Chang DW, Xing Z, Pan Y, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21(14):3704–14.
Dillon CP, Oberst A, Weinlich R, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1(5):401–7.
O’Donnell MA, Perez-Jimenez E, Oberst A, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13(12):1437–42.
•• Oberst A, Dillon CP, Weinlich R, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–7. Recent study showing the effect of caspase-8 catalytic activity during programmed cell death.
Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95.
Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23.
He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11.
Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21.
Smith CCT, Davidson SM, Lim SY, et al. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–33.
•• Oerlemans MIFJ, Liu J, Arslan F, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107(4):270. Recent in vivo study showing a positive effect of necrostatin during myocardial ischemia/reperfusion injury.
Zhu S, Zhang Y, Bai G, Li H. Necrostatin-1 ameliorates symptoms in R6/2 transgenic mouse model of Huntington’s disease. Cell Death Dis. 2011;2:e115.
Davis CW, Hawkins BJ, Ramasamy S, et al. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic Biol Med. 2010;48:306–17.
Zhang D-W, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science (New York, NY). 2009;325(5938):332–6.
Nevins JR, Leone G, DeGregori J, Jakoi L. Role of the Rb/E2F pathway in cell growth control. J Cell Physiol. 1997;173:233–6.
Shan B, Lee WH. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14:8166–73.
Kirshenbaum LA, Abdellatif M, Chakraborty S, Schneider MD. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev Biol. 1996;179:402–11.
Shaw J, Yurkova N, Zhang T, et al. Antagonism of E2F-1 regulated Bnip3 transcription by NF-kappaB is essential for basal cell survival. Proc Natl Acad Sci USA. 2008;105(52):20734–9.
Yurkova N, Shaw J, Blackie K, et al. The cell cycle factor E2F-1 activates Bnip3 and the intrinsic death pathway in ventricular myocytes. Circ Res. 2008;102(4):472–9.
Disclosure
W. Mughal has received a grant from Manitoba Health Research Council; R. Dhingra has received a grant from Manitoba Health Research Council; L. A. Kirshenbaum has received a grant from Canadian Institutes of Health Research and Heart and Stroke Foundation, and is a Canada Research Chair in Molecular Cardiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mughal, W., Dhingra, R. & Kirshenbaum, L.A. Striking a Balance: Autophagy, Apoptosis, and Necrosis in a Normal and Failing Heart. Curr Hypertens Rep 14, 540–547 (2012). https://doi.org/10.1007/s11906-012-0304-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-012-0304-5