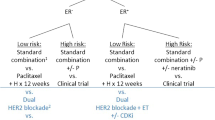
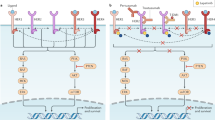
Opinion statement
The management of patients with HER2+ breast cancer has evolved significantly over the preceding decades. HER2 targeting strategies have advanced beyond focusing on the receptor alone to encompass a range of approaches. Current standard of care practices in these patients relies upon dual HER2 blockade with trastuzumab and pertuzumab in the adjuvant and metastatic settings. T-DM1 has proven particularly efficacious in patients with residual disease status post neoadjuvant therapy, with additional therapies approved in the subsequent lines to address recurrent and resistant disease. Advances continue to be made in HER2+ breast cancer with multiple novel agents on the horizon, employing diverse mechanisms of action that are described in this review.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551.
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51. https://doi.org/10.3322/caac.21583.
Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16(10):5276–87. https://doi.org/10.1128/mcb.16.10.5276.
Natali PG, Nicotra MR, Bigotti A, Venturo I, Slamon DJ, Fendly BM, et al. Expression of the p185 encoded by HER2 oncogene in normal and transformed human tissues. Int J Cancer. 1990;45(3):457–61. https://doi.org/10.1002/ijc.2910450314.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. https://doi.org/10.1056/NEJM200103153441101.
Montemurro F, Donadio M, Clavarezza M, Redana S, Jacomuzzi ME, Valabrega G, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. Oncologist. 2006;11(4):318–24. https://doi.org/10.1634/theoncologist.11-4-318.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. https://doi.org/10.1200/JCO.2002.20.3.719.
Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19(53):6102–14. https://doi.org/10.1038/sj.onc.1203973.
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–6. https://doi.org/10.1158/0008-5472.CAN-08-4597.
Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–71. https://doi.org/10.1016/S1470-2045(13)70130-X.
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. https://doi.org/10.1056/NEJMoa1413513.
• von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–31. https://doi.org/10.1056/NEJMoa1703643 This study was a randomized placebo-controlled trial that found longer invasive-disease-free survival when adding pertuzumab to trastuzumab for early breast cancer.
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43. https://doi.org/10.1056/NEJMoa064320.
Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. https://doi.org/10.1016/S1470-2045(12)70432-1.
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128(2):347–56. https://doi.org/10.1007/s10549-010-1090-x.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28. https://doi.org/10.1056/NEJMoa1814017.
Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557–64. https://doi.org/10.1001/jamaoncol.2016.0237.
Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–700. https://doi.org/10.1016/S1470-2045(17)30717-9.
Statler AB, Hobbs BP, Wei W, Gupta A, Blake CN, Nahleh ZA. Real-world treatment patterns and outcomes in HR+/HER2+ metastatic breast cancer patients: a national cancer database analysis. Sci Rep. 2019;9(1):18126. https://doi.org/10.1038/s41598-019-54402-9.
Giuliano M, Hu H, Wang YC, Fu X, Nardone A, Herrera S, et al. Upregulation of ER signaling as an adaptive mechanism of cell survival in HER2-positive breast tumors treated with anti-HER2 therapy. Clin Cancer Res. 2015;21(17):3995–4003. https://doi.org/10.1158/1078-0432.CCR-14-2728.
• Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24(5):638–46. https://doi.org/10.1038/s41591-018-0007-9 The study provided an in-depth characterization of the exon-20 mutant HER2 receptor and its sensitivity to poziotinib.
Kim JY, Lee E, Park K, Jung HH, Park WY, Lee KH, et al. Molecular alterations and poziotinib efficacy, a pan-HER inhibitor, in human epidermal growth factor receptor 2 (HER2)-positive breast cancers: combined exploratory biomarker analysis from a phase II clinical trial of poziotinib for refractory HER2-positive breast cancer patients. Int J Cancer. 2019;145(6):1669–78. https://doi.org/10.1002/ijc.32188.
Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58. https://doi.org/10.1186/s12943-018-0782-4.
Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1(5):445–57. https://doi.org/10.1016/s1535-6108(02)00072-7.
• Murthy R, Borges VF, Conlin A, Chaves J, Chamberlain M, Gray T, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(7):880–8. https://doi.org/10.1016/S1470-2045(18)30256-0 This phase 1b study reported the low toxicity profile of the highly specific tyrosine kinase inhibitor tucatinib.
•• Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. https://doi.org/10.1056/NEJMoa1914609 Randomized, double-blind trial that reported increased progression-free survival and overall survival by adding tucatinib to conventional therapy in metastatic HER2+ breast cancer, including a 1-year progression-free survival in 24% of patients with brain metastasis.
Moulder SL, Borges VF, Baetz T, McSpadden T, Fernetich G, Murthy RK, et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2(+)-advanced solid tumors, with an expansion cohort in HER2(+) metastatic breast cancer (MBC). Clin Cancer Res. 2017;23(14):3529–36. https://doi.org/10.1158/1078-0432.CCR-16-1496.
Dinkel V, Anderson D, Winski S, Winkler J, Koch K, Lee PA. ARRY-380, a potent, small molecule inhibitor of ErbB2, increases survival in intracranial ErbB2+xenograft models in mice. Cancer Res. 2012;72. https://doi.org/10.1158/1538-7445.Am2012-852.
Borges VF, Ferrario C, Aucoin N, Falkson C, Khan Q, Krop I, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer a phase 1b clinical trial. Jama Oncol. 2018;4(9):1214–20. https://doi.org/10.1001/jamaoncol.2018.1812.
Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108(12):5021–6. https://doi.org/10.1073/pnas.1016140108.
Trowe T, Boukouvala S, Calkins K, Cutler RE, Fong R, Funke R, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008;14(8):2465–75. https://doi.org/10.1158/1078-0432.Ccr-07-4367.
Wetterskog D, Shiu KK, Chong I, Meijer T, Mackay A, Lambros M, et al. Identification of novel determinants of resistance to lapatinib in ERBB2-amplified cancers. Oncogene. 2014;33(8):966–76. https://doi.org/10.1038/onc.2013.41.
Zhang K, Hong RX, Kaping L, Xu F, Xia W, Qin G, et al. CDK4/6 inhibitor palbociclib enhances the effect of pyrotinib in HER2-positive breast cancer. Cancer Lett. 2019;447:130–40. https://doi.org/10.1016/j.canlet.2019.01.005.
Wang Y, Jiang T, Qin Z, Jiang J, Wang Q, Yang S, et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol. 2019;30(3):447–55. https://doi.org/10.1093/annonc/mdy542.
Ma F, Ouyang QC, Li W, Jiang ZF, Tong ZS, Liu YJ, et al. Pyrotinib or lapatinib combined with capecitabine in HER2? Positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol. 2019;37(29):2610. https://doi.org/10.1200/Jco.19.00108.
Nagano M, Kohsaka S, Ueno T, Kojima S, Saka K, Iwase H, et al. High-throughput functional evaluation of variants of unknown significance in ERBB2. Clinical Cancer Research. 2018;24(20):5112–22.https://doi.org/10.1158/1078-0432.Ccr-18-0991
•• Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers (vol 554, pg 189, 2018). Nature. 2019;566(7745):E11–E2. https://doi.org/10.1038/s41586-019-0974-0 Novel genomically-selected and multi-histology trial, which sought to prospectively define HER2 and HER3 mutants and their response to neratinib.
Cha MY, Lee KO, Kim M, Song JY, Lee KH, Park J, et al. Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int J Cancer. 2012;130(10):2445–54. https://doi.org/10.1002/ijc.26276.
Robichaux JP, Elamin YY, Vijayan RSK, Nilsson MB, Hu LM, He JQ, et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell. 2019;36(4):444. https://doi.org/10.1016/j.ccell.2019.09.001.
Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–14. https://doi.org/10.1038/onc.2008.432.
Kim JY, Lee E, Park K, Jung HH, Park WY, Lee KH, et al. Molecular alterations and poziotinib efficacy, a pan-HER inhibitor, in human epidermal growth factor receptor 2 (HER2)-positive breast cancers: combined exploratory biomarker analysis from a phase II clinical trial of poziotinib for refractory HER2-positive breast cancer patients. Int J Cancer. 2019;145(6):1669–78. https://doi.org/10.1002/ijc.32188.
Park YH, Lee KH, Sohn JH, Lee KS, Jung KH, Kim JH, et al. A phase II trial of the pan-HER inhibitor poziotinib, in patients with HER2-positive metastatic breast cancer who had received at least two prior HER2-directed regimens: results of the NOV120101–203 trial. Int J Cancer. 2018;143(12):3240–7. https://doi.org/10.1002/ijc.31651.
Pandey A, Brufsky AM. Metastatic breast cancer patient with activating HER2 Exon 20 insertion mutation with response to poziotinib: case report of compassionate drug use. Clin Breast Cancer. 2019;19(1):E7–E11. https://doi.org/10.1016/j.clbc.2018.09.010.
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–108. https://doi.org/10.1158/1078-0432.CCR-15-2822.
• Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–21. https://doi.org/10.1056/NEJMoa1914510 Single-arm study that found an impressive response rate of 60.9% in a heavily pretreated population and duration of response of 14.8 months.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. https://doi.org/10.1056/NEJMoa1209124.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. https://doi.org/10.1111/cas.12966.
Modi S, Tsurutani J, Tamura K, Park H, Sagara Y, Murthy R, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer: Updated results of a large phase 1 study. Cancer Res. 2019;79(4). https://doi.org/10.1158/1538-7445.Sabcs18-P6-17-02.
Shanu Modi SO, Caleb C, Lee KW, Saxena K, Cameron DA. A phase 3, multicenter, randomized, open-label trial of trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator’s choice in HER2-low breast cancer (DESTINY-BREAST04). San Antonio: Presented at the 2019 San Antonio Breast Cancer Symposium; 2019.
Fabrice André JS, Lee C, Wang K, Krop IE. [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator’s choice of treatment in subjects with HER2-positive, unresectable and/or metastatic breast cancer who previously received T-DM1: a randomized, phase 3 trial (DESTINY-BREAST02). San Antonio: Presented at the 2019 San Antonio breast Cancer symposium. p. 2019.
Javier Cortés JS, Lee C, Zhang Y, Verma S. [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs ado-trastuzumab emtansine (T-DM1) in subjects with HER2-positive, unresectable and/or metastatic breast cancer who previously received trastuzmab and a taxane: a phase 3, randomized trial (DESTINY-BREAST03). San Antonio: Presented at the 2019 San Antonio breast Cancer symposium. p. 2019.
Dokter W, Ubink R, van der Lee M, van der Vleuten M, van Achterberg T, Jacobs D, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13(11):2618–29. https://doi.org/10.1158/1535-7163.MCT-14-0040-T.
van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol Cancer Ther. 2015;14(3):692–703. https://doi.org/10.1158/1535-7163.MCT-14-0881-T.
Menderes G, Bonazzoli E, Bellone S, Black J, Predolini F, Pettinella F, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows antitumor activity in uterine and ovarian carcinosarcoma with HER2/Neu expression. Clin Cancer Res. 2017;23(19):5836–45. https://doi.org/10.1158/1078-0432.CCR-16-2862.
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–35. https://doi.org/10.1016/S1470-2045(19)30328-6.
Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–42. https://doi.org/10.1038/bjc.2017.367.
Humphreys RC, Kirtely J, Hewit A, Biroc S, Knudsen N, Skidmore L, et al. Site specific conjugation of ARX-788, an antibody drug conjugate (ADC) targeting HER2, generates a potent and stable targeted therapeutic for multiple cancers. Cancer Res. 2015;75. https://doi.org/10.1158/1538-7445.Am2015-639.
Barok M, Le Joncour V, Martins A, Isola J, Salmikangas M, Laakkonen P, et al. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer. Cancer Lett. 2020;473:156–63. https://doi.org/10.1016/j.canlet.2019.12.037.
Xichun Hu JZ, Ji D, Xia G, Ji Y, Xiong G, Liang X. A phase I study of ARX788, a HER2-targeting antibody-drug conjugate, in patients with metastatic HER2-positive breast cancer. San Antonio: Poster at the 2019 San Antonio breast Cancer symposium. p. 2019.
Albanell J, Codony J, Rovira A, Mellado B, Gascon P. Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4. Adv Exp Med Biol. 2003;532:253–68. https://doi.org/10.1007/978-1-4615-0081-0_21.
Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61. https://doi.org/10.1093/annonc/mdx002.
Hope S, Rugo S-AI, Wright GLS, Escriva-de-Romani S, DeLaurentiis M, Cortes J, et al. Phase 3 SOPHIA study of margetuximab + chemotherapy vs trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: second interi overall survival analysis: Presented at the 2019 ASCO conference. This study highlights the efficacy of this bispecific antibody in prolonging progression-free survival
Hope S, Rugo S-AI, Wright GLS, Escriva-de-Romani S, Delaurentiis M, Cortes J, et al. Phase 3 SOPHIA study of margetuximab + chemotherapy vs trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: second interi overall survival analysis. San Antonio: Presented at the 2019 San Antonio breast Cancer symposium. p. 2019.
Grabert RC, Cousens LP, Smith JA, Olson S, Gall J, Young WB, et al. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res. 2006;12(2):569–76. https://doi.org/10.1158/1078-0432.CCR-05-2005.
Han H, Ma J, Zhang K, Li W, Liu C, Zhang Y, et al. Bispecific anti-CD3 x anti-HER2 antibody mediates T cell cytolytic activity to HER2-positive colorectal cancer in vitro and in vivo. Int J Oncol. 2014;45(6):2446–54. https://doi.org/10.3892/ijo.2014.2663.
Thakur A, Norkina O, Lum LG. In vitro synthesis of primary specific anti-breast cancer antibodies by normal human peripheral blood mononuclear cells. Cancer Immunol Immunother. 2011;60(12):1707–20. https://doi.org/10.1007/s00262-011-1056-9.
Ma J, Han H, Liu D, Li W, Feng H, Xue X, et al. HER2 as a promising target for cytotoxicity T cells in human melanoma therapy. PLoS One. 2013;8(8):e73261. https://doi.org/10.1371/journal.pone.0073261.
Lum LG, Thakur A, Al-Kadhimi Z, Colvin GA, Cummings FJ, Legare RD, et al. Targeted T-cell therapy in stage IV breast cancer: a phase I clinical trial. Clin Cancer Res. 2015;21(10):2305–14. https://doi.org/10.1158/1078-0432.CCR-14-2280.
De Nardis C, Hendriks LJA, Poirier E, Arvinte T, Gros P, Bakker ABH, et al. A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. J Biol Chem. 2017;292(35):14706–17. https://doi.org/10.1074/jbc.M117.793497.
Jonna S, Feldman RA, Swensen J, Gatalica Z, Korn WM, Borghaei H, et al. Detection of NRG1 gene fusions in solid tumors. Clin Cancer Res. 2019;25(16):4966–72. https://doi.org/10.1158/1078-0432.CCR-19-0160.
MCLA-128 Fights NRG1 fusion-positive cancers. Cancer Discov. 2019;9(12):1636. https://doi.org/10.1158/2159-8290.CD-NB2019-128.
Back J, Wermke M, Macoin J, Croset A, Kauh JS, Reddy V. GBR1302: Effect of CD3-HER2, a bispecific T cell engager antibody, in trastuzumab-resistant cancers. J Clin Oncol. 2018;36(15). https://doi.org/10.1200/JCO.2018.36.15_suppl.12053.
ZW25 Effective in HER2-positive cancers. Cancer Discov. 2019;9(1):8. https://doi.org/10.1158/2159-8290.CD-NB2018-162.
Beeram M, Hamilton E, Hanna D, Ajani J, Murphy MB, Bendell J, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in HER2-expressing gastroesophageal and other cancers. Eur J Cancer. 2018;103:E17.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5). https://doi.org/10.1093/jnci/dju055.
Metzger SMO, Loibl S, Mundhenke C, Seiler S, Valagussa P, Lim E, et al. Abstract OT3–02-07: PATINA: a randomized, open label, phase III trial to evaluate the efficacy and safety of palbociclib + anti-HER2 therapy + endocrine therapy (ET) vs. anti-HER2 therapy + ET after induction treatment for hormone receptor positive (HR+)/HER2-positive metastatic breast cancer (MBC). Cancer Res. 2019;79(4). https://doi.org/10.1158/1538-7445.SABCS18-OT3-02-07 This is the first study evaluating a CDK4/6 inhibitor in combination with anti-HER2 therapy for management of metastatic HER2+/HR+ disease.
Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17(15):5132–9. https://doi.org/10.1158/1078-0432.CCR-11-0072.
Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, et al. AVEREL: a randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31(14):1719–25. https://doi.org/10.1200/JCO.2012.44.7912.
Miller K, Cortes J, Hurvitz SA, Krop IE, Tripathy D, Verma S, et al. HERMIONE: a randomized phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naive, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer. 2016;16:352. https://doi.org/10.1186/s12885-016-2385-z.
•• Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. https://doi.org/10.1056/NEJMoa1809615 This trial reported benefit in outcomes with the addition of a checkpoint inhibitor to chemotherapy in the first line in PD-L1 positive, metastatic triple-negative breast cancer.
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–86. https://doi.org/10.1007/s10549-017-4537-5.
• Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20(3):371–82. https://doi.org/10.1016/S1470-2045(18)30812-X The study found that only PD-L1 positive patients had a durable response to pembrolizumab and trastuzumab.
LA Emens FE, Beresford M, Saura C, De Laurentiis M, Kim S-B, Im S-A, et al. Abstract PD3–01: results from KATE2, a randomized phase 2 study of atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC). Cancer Res. 2019;79(4). https://doi.org/10.1158/1538-7445.SABCS18-PD3-01.
An investigator-initiated, non-randomised, phase II study of combination CTLA-4 and PD-L1 blockade in combination with HER2 blockade in advanced HER2-positive breast cancers that have progressed on prior trastuzumab-based therapy. Current clinical trials DIAmOND BCT 1703.
The AVIATOR study: trastuzumab and vinorelbine with avelumab OR avelumab & utomilumab in advanced HER2+ breast cancer Clinicaltrials.gov2018 [updated 01.22.2020. Available from: https://clinicaltrials.gov/ct2/show/NCT03414658.
Krasniqi E, Barchiesi G, Pizzuti L, Mazzotta M, Venuti A, Maugeri-Sacca M, et al. Immunotherapy in HER2-positive breast cancer: state of the art and future perspectives. J Hematol Oncol. 2019;12(1):111. https://doi.org/10.1186/s13045-019-0798-2.
Berzofsky JA, Terabe M, Trepel JB, Pastan I, Stroncek DF, Morris JC, et al. Cancer vaccine strategies: translation from mice to human clinical trials. Cancer Immunol Immunother. 2018;67(12):1863–9. https://doi.org/10.1007/s00262-017-2084-x.
Vaccine therapy, trastuzumab, and vinorelbine in treating patients with locally recurrent or metastatic breast cancer Clinicaltrials.gov [updated 09/18/2020. Available from: https://clinicaltrials.gov/ct2/show/NCT00266110.
Chen F, Ma K, Madajewski B, Zhuang L, Zhang L, Rickert K, et al. Ultrasmall targeted nanoparticles with engineered antibody fragments for imaging detection of HER2-overexpressing breast cancer. Nat Commun. 2018;9(1):4141. https://doi.org/10.1038/s41467-018-06271-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Juan Luis Gomez Marti declares that he has no conflict of interest. Tara Hyder declares that she has no conflict of interest. Azadeh Nasrazadani declares that she has no conflict of interest. Adam M. Brufsky has received compensation from Roche, Novartis, Daiichi Sankyo, AstraZeneca, Pfizer, and Puma for service as a consultant.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Marti, J.L.G., Hyder, T., Nasrazadani, A. et al. The Evolving Landscape of HER2-Directed Breast Cancer Therapy. Curr. Treat. Options in Oncol. 21, 82 (2020). https://doi.org/10.1007/s11864-020-00780-6
Published:
DOI: https://doi.org/10.1007/s11864-020-00780-6