Abstract
Objective
Bariatric procedures that exclude the proximal small intestine lead to significant weight loss which is probably mediated by changes in hormones that alter appetite, such as peptide YY (PYY), ghrelin, cholecystokinin (CCK), and leptin. Here, the effect of the non-surgical duodenal-jejunal bypass liner (DJBL) on concentrations of hormones implicated in appetite control was investigated.
Subjects
A two-center prospective study was conducted between January and December 2010. Seventeen obese subjects with type 2 diabetes were treated with the DJBL for 24 weeks. Fasting concentrations of leptin and meal responses of plasma PYY, CCK, and ghrelin were determined prior to and after implantation of the DJBL.
Results
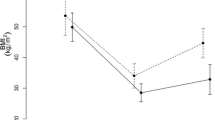
At baseline, subjects had an average body weight of 116.0 ± 5.8 kg. One week after implantation, subjects had lost 4.3 ± 0.6 kg (p < 0.01), which progressed to 12.7 ± 1.3 kg at week 24 (p < 0.01). Postprandial concentrations of PYY and ghrelin increased (baseline vs. week 1 vs. week 24 PYY: 2.6 ± 0.2 vs. 4.1 ± 0.4 vs. 4.1 ± 0.7 nmol/L/min and ghrelin: 7.8 ± 1.8 vs. 11.0 ± 1.8 vs. 10.6 ± 1.8 ng/mL/min, all p < 0.05). In parallel, the CCK response decreased (baseline vs. week 1 vs. week 24: 434 ± 51 vs. 229 ± 52 vs. 256 ± 51pmol/L/min, p < 0.01). Fasting leptin concentrations also decreased (baseline vs. week 24: 98 ± 17 vs. 53 ± 10 ng/mL, p < 0.01).
Conclusions
DJBL treatment induces weight loss paralleled by changes in concentrations of hormones involved in appetite control.

Similar content being viewed by others
References
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Kaplan LM. Pharmacological therapies for obesity. Gastroenterol Clin North Am. 2005;34(1):91–104.
Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288(22):2793–6.
Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93(2):210–5.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5.
Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128(1):175–91.
Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132(6):2116–30.
Michalakis K, le Roux C. Gut hormones and leptin: impact on energy control and changes after bariatric surgery—what the future holds. Obes Surg. 2012;22(10):1648–57.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5.
Zwirska-Korczala K, Konturek SJ, Sodowski M, Wylezol M, Kuka D, Sowa P, et al. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol : Off J Polish Physiol Soc. 2007;58 Suppl 1:13–35.
le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3–8.
Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–9.
Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology. 2003;124(5):1532–5.
Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50(9):1511–25.
le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108–14.
Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110(4):571–84.
Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234–41.
Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes. 2011;35(2):153–66.
Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8.
Tymitz K, Engel A, McDonough S, Hendy MP, Kerlakian G. Changes in ghrelin levels following bariatric surgery: review of the literature. Obes Surg. 2011;21(1):125–30.
Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–95.
Escalona A, Pimentel F, Sharp A, Becerra P, Slako M, Turiel D, et al. Weight loss and metabolic improvement in morbidly obese subjects implanted for 1 year with an endoscopic duodenal-jejunal bypass liner. Ann Surg. 2012;255(6):1080–5.
Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, et al. A multicenter, randomized efficacy study of the endobarrier gastrointestinal liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251(2):236–43.
de Jonge C, Rensen SS, Verdam FJ, Vincent RP, Bloom SR, Buurman WA, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg. 2013;23(9):1354–60.
Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2(8571):1300–4.
van Dielen FM, Buurman WA, Hadfoune M, Nijhuis J, Greve JW. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89(8):4062–8.
Miras AD, le Roux CW. Bariatric surgery and taste: novel mechanisms of weight loss. Curr Opin Gastroenterol. 2010;26(2):140–5.
Bueter M, le Roux CW. Gastrointestinal hormones, energy balance and bariatric surgery. Int J Obes. 2011;35 Suppl 3:S35–9.
Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117(1):13–23.
Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–8.
Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev. 2006;7(2):163–82.
Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg. 2003;138(4):389–96.
Sato T, Nakamura Y, Shiimura Y, Ohgusu H, Kangawa K, Kojima M. Structure, regulation and function of ghrelin. J Biochem. 2012;151(2):119–28.
Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity. 2010;18(2):221–9.
Maljaars PW, Symersky T, Kee BC, Haddeman E, Peters HP, Masclee AA. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int J Obes. 2008;32(11):1633–9.
Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav. 2008;95(3):271–81.
Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19(1):8–12.
Verhulst PJ, Depoortere I. Ghrelin’s second life: from appetite stimulator to glucose regulator. World J Gastroenterol. 2012;18(25):3183–95.
Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86(10):4753–8.
Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S37–50.
Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest. 2012;122(12):4667–74.
de Moura EG, Orso IR, Martins Bda C, Lopes GS, de Oliveira SL, Galvao-Neto Mdos P, et al. Improvement of insulin resistance and reduction of cardiovascular risk among obese patients with type 2 diabetes with the duodenojejunal bypass liner. Obes Surg. 2011;21(7):941–7.
Evans S, Pamuklar Z, Rosko J, Mahaney P, Jiang N, Park C, et al. Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction. Surg Endosc. 2012;26(4):1086–94.
Acknowledgments
The authors would like to thank the subjects contributing to this trial; the trial nurses Y. Wils and R. Nelissen, and the students who helped conducting this research: G. Latten, N. Geubbels, M. de Wolf, T. van der Horst, R. Erbil, B. van der Putten, H. D’Agnolo, S. Peeters Weem, and M.A. Joosten; B. Winkens for statistical assistance; Dr. R.J. de Ridder, Dr. G.H. Koek, and Dr. C.M. Bakker for their help with the DJBL procedures; Prof. Dr. A.A. Masclee, Dr. J. Maljaars, and Y. Slaats for their help regarding the study design, and Dr. I.C. Arts and E. Theunisz for the Luminex analyses.
Clinical Trial Registration Number: NCT00985114
Conflict of Interest
N.D.B. and J.W.M.G. disclose the following financial relationships relevant to this publication. N.D.B. received an open research grant from GI dynamics. J.W.M.G. received consultancy fees an open research grant and support for travel to meetings for the study or other purposes from GI dynamics. All other authors have no conflicts of interest relevant to this article.
Grant Information
C.J., N.D.B. and J.W.M.G. disclose the following financial relationships relevant to this publication. C.J. received support for travel to meetings for the study or other purposes from GI dynamics. N.D.B. received an open research grant from GI dynamics. J.W.M.G. received consultancy fees an open research grant and support for travel to meetings for the study or other purposes from GI dynamics. All other authors have no conflicts of interest relevant to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Jonge, C., Rensen, S.S., Verdam, F.J. et al. Impact of Duodenal-Jejunal Exclusion on Satiety Hormones. OBES SURG 26, 672–678 (2016). https://doi.org/10.1007/s11695-015-1889-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1889-y