Abstract
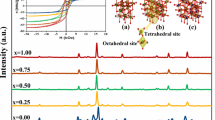
Silico-ferrite of calcium (SFC) is a key intermediate phase in the sintering process of fine iron ores, and SiO2 plays an important role in the formation of SFC. In this work, the crystal structure stability of SFC synthesized at 1473 K (1200 °C) has been determined by X-ray diffraction, field-emission scanning electron microscopy, and X-ray absorption spectra. Synthesis of SFC was carried out under air at 1473 K (1200 °C) by mixing different amounts of SiO2 with Fe2O3 and CaCO3. The results show that the maximum solid solubility of SiO2 in the crystal structure of SFC does not exceed 6.11 wt pct at 1473 K (1200 °C); under these conditions, Fe2O3 begins to appear. The process of Si solution is closely related to the presence of a Ca channel composed of Ca octahedron in the crystal structure of SFC based on the results from the measurements of Ca K-edge X-ray absorption spectra. Si mainly occupies the center positions of the upper and lower tetrahedron adjacent to Ca channel. The length of Ca-Ca bond in Ca channel increases with the increasing of Si content. The crystal structure stability of SFC may be related to the structure of the Ca channel.









Similar content being viewed by others
References
T. Ikeda, K. Inoue, T. Uenaka, and M. Kanemoto: Tetsu-to-Hagané, 1981, vol. 67, pp. 726–35.
K. Inoue and T. Ikeda: Tetsu-to-Hagané, 1982, vol. 68, pp. 2190–99.
J.D.G. Hamilton, B.F. Hoskins, W.G. Mumme, W.E. Borbidge, and M.A. Montague: Neues Jahrb. Miner. Abh., 1989, vol. 161, pp. 1–26.
M.I. Pownceby, J.M.F. Clout, and M.J. Fisher-White: Trans. Inst. Min. Metall. (Sect. C), 1998, vol. 107, pp. C1–10.
M.I. Pownceby and T.R.C. Patrick: Eur. J. Mineral., 2000, vol. 12, pp. 455–68.
M.I. Pownceby and J.M.F. Clout: Trans. Inst. Min. Metall. (Sect. C), 2000, vol. 109, pp. C36–48.
T.R.C. Patrick and M.I. Pownceby: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 79–89.
N.V.Y. Scarlett, M.I. Pownceby, I.C. Madsen, and A.N. Christensen: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 929–36.
N.V.Y. Scarlett, I.C. Madsen, M.I. Pownceby, and A.N. Christensen: J. Appl. Crystallogr., 2004, vol. 37, pp. 362–68.
N.A.S. Webster, M.I. Pownceby, I.C. Madsen, and J.A. Kimpton: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 1344–57.
N.A.S. Webster, M.I. Pownceby and I.C. Madsen: ISIJ International, 2013, vol. 53(8), pp. 1334–40.
D.H. Lister and F.P. Glasser: Brit. Ceram. Trans. J., 1967, vol. 66, pp. 293–305.
W.G. Mumme: Neues Jahrb. Miner. Abh., 2003, vol. 178, pp. 307–35.
X. Ding and X.-M. Guo: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 1221–31.
B. Ravel and M. Newville: J. Synchrotron Rad., 2005, vol. 12, pp. 537–41.
L.-W. Du, S. Bian, B.-D. Gou, Y. Jiang, J. Huang, Y.-X. Gao, Y.-D. Zhao, W. Wen, T.-L. Zhang, and K. Wang: Crystal Growth & Design, 2013, vol. 13(7), pp. 3103–9.
K. Asokan, J.C. Jan, J.W. Chiou, W.F. Pong, M.-H. Tsai, Y.K. Chang, Y.Y. Chen, H.H. Hsieh, H.-J. Lin, Y.W. Yang, L.J. Lai, and I.N. Lin: Journal of Solid State Chemistry, 2004, vol. 177, pp. 2639–43.
D.R. Neuville, L. Cormier, A.-M. Flank, V. Briois, and D. Massiot: Chemical Geology, 2004, vol. 213, pp. 153–63.
O Haas, Chr Ludwig, U Bergmann, RN Singh, A Braun, T Graule (2011) Journal of Solid State Chemistry. 184, 3163-71.
I. Tanaka and T. Mizoguchi: J. Phys.: Condens. Matter, 2009, vol. 21, pp. 1–9.
A. Bianconi: XANES spectroscopy, 1988, pp. 573–662.
D. Eichert, M. Salome, M. Banu, J. Susini, and C. Rey: Spectrochim. Acta B: Atom. Spectrosc, 2005, vol. 60, pp. 850–8.
E. Paris and T.A. Tyson: Phys. Chem. Minerals, 1994, vol. 21, pp. 299–308.
B. Gilbert, B.H. Frazer, A. Belz, P.G. Conrad, K.H. Nealson, D. Haskel, J.C. Lang, G. Srajer, and G.D. Stasio: J. Phys. Chem. A, 2003, vol. 107, pp. 2839–47.
K. Brandenburg: DIAMOND, Crystal Impact GbR, Bonn, Germany, 1999.
Acknowledgments
The authors thank the National Natural Science Foundation of China for providing the financial support (Grants U146020005 and 51374017).
The XANES beam time was granted by 4B7A end-station of Beijing Synchrotron Radiation Facility, Institute of High Energy Physics Chinese Academy of Sciences. The staff members of 4B7A are acknowledged for their support in undertaking these measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 6, 2014.
Rights and permissions
About this article
Cite this article
Ding, X., Guo, XM., Ma, CY. et al. Effect of SiO2 on the Crystal Structure Stability of SFC at 1473 K (1200 °C). Metall Mater Trans B 46, 1146–1153 (2015). https://doi.org/10.1007/s11663-015-0313-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0313-2