Abstract
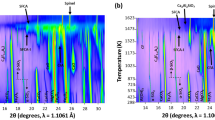
The formation of silico-ferrite of calcium and aluminum (SFCA) and SFCA-I iron ore sinter phases during heating and cooling of synthetic iron ore sinter mixtures in the range 298 K to 1623 K (25 °C to 1350 °C) and at oxygen partial pressure of 5 × 10−3 atm has been characterized using in situ synchrotron X-ray diffraction. SFCA and SFCA-I are the key bonding phases in iron ore sinter, and an improved understanding of their formation mechanisms may lead to improved efficiency of industrial sintering processes. During heating, SFCA-I formation at 1327 K to 1392 K (1054 °C to 1119 °C) (depending on composition) was associated with the reaction of Fe2O3, 2CaO·Fe2O3, and SiO2. SFCA formation (1380 K to 1437 K [1107 °C to 1164 °C]) was associated with the reaction of CaO·Fe2O3, SiO2, and a phase with average composition 49.60, 9.09, 0.14, 7.93, and 32.15 wt pct Fe, Ca, Si, Al, and O, respectively. Increasing Al2O3 concentration in the starting sinter mixture increased the temperature range over which SFCA-I was stable before the formation of SFCA, and it stabilized SFCA to a higher temperature before it melted to form a Fe3O4 + melt phase assemblage (1486 K to 1581 K [1213 °C to 1308 °C]). During cooling, the first phase to crystallize from the melt (1452 K to 1561 K [1179 °C to 1288 °C]) was an Fe-rich phase, similar in composition to SFCA-I, and it had an average composition 58.88, 6.89, 0.82, 3.00, and 31.68 wt pct Fe, Ca, Si, Al, and O, respectively. At lower temperatures (1418 K to 1543 K [1145 °C to 1270 °C]), this phase reacted with melt to form SFCA. Increasing Al2O3 increased the temperature at which crystallization of the Fe-rich phase occurred, increased the temperature at which crystallization of SFCA occurred, and suppressed the formation of Fe2O3 (1358 K to 1418 K [1085 °C to 1145 °C]) to lower temperatures.










Similar content being viewed by others
References
P.R. Dawson, J. Ostwald, and K.M. Hayes: T. I. Min. Metall. C, 1985, vol. 94, pp. 71–8.
L-H. Hsieh and J.A. Whiteman: ISIJ Int., 1989, vol. 29, pp. 24–32.
L.-H. Hsieh and J.A. Whiteman: ISIJ Int., 1989, vol. 29, pp. 625–34.
T.R.C. Patrick and R.R. Lovel: ISIJ Int., 2001, vol. 41, pp. 128–35.
T. Mukherjee and J.A. Whiteman: Ironmaking Steelmaking, 1985, vol. 12, pp. 151–55.
J. Ostwald: BHP Tech. Bull., 1981, vol. 25, pp. 13–20.
N.J. Bristow and A.G. Waters: T. I. Min. Metall. C, 1991, vol. 100, pp. 1–10.
I. Shigaki, M. Sawada, and N. Gennai: T. Iron Steel I. Japan, 1986, vol. 26, pp. 503–11.
C.E. Loo, K.T. Wan, and V.R. Howes: Ironmaking Steelmaking, 1988, vol. 15, pp. 279–85.
C. Chen, L. Zhang, L. Lu, and S. Sun: ISIJ Int., 2010, vol. 50, pp. 1532–1.
J.D.G. Hamilton, B.F. Hoskins, W.G. Mumme, W.E. Borbidge, and M.A. Montague: Neues Jahrb. Miner. Abh., 1989, vol. 161, pp. 1–26.
J. Hancart, V. Leroy, and A. Bragard: C.N.R.M. Report, 1967, DS 24/67, pp. 3–7.
S.N. Ashan, T. Mukkerjee, and J.A. Whiteman: Ironmaking Steelmaking, 2003, vol. 10, pp. 54–64.
T.R.C. Patrick and M.I. Pownceby: Metall. Mater. Trans. B, 2001, vol. 32B, pp. 1–11.
W.G. Mumme, J.M.F. Clout, and R.W. Gable: Neues Jahrb. Miner. Abh., 1998, vol. 173, pp. 93–117.
J. McAndrew and J.M.F. Clout: Proc. of the 4th China-Australia Symposium on the Technology of Feed Preparation for Ironmaking, 1993, Dampier, Australia, pp. 1–15.
W.G. Mumme: Neues Jahrb. Miner. Abh., 2003, vol. 178, pp. 307–35.
D.H. Lister and F.P. Glasser: Brit. Ceram. Trans. J., 1967, vol. 66, pp. 293–305.
T. van den Berg and J.P.R. de Villiers: Ninth International Congress for Applied Mineralogy, Brisbane, QLD, September 8–10, 2008, pp. 713–17.
T. van den Berg and J.P.R. de Villiers: T. I. Min. Metall. C, 2009, vol. 118, pp. 214–21.
J.P.R. de Villiers and S.M.C. Verryn: Ninth International Congress for Applied Mineralogy, Brisbane, QLD, September 8–10, 2008, pp. 265–75.
Y. Hida, M. Sasaki, K. Sato, M. Kagawa, T. Miyazaki, H. Soma, H. Naito, and M. Taniguchi: Nippon Steel Tech. Report, 1987, vol. 35, pp. 59–67.
Y. Hida, J. Okazaki, K. Itoh, and M. Sasaki: Tetsu-To-Hagané, 1987, vol. 73, pp. 1893–900.
M. Sasaki and Y. Hida: Tetsu-To-Hagane, 1982, vol. 68, pp. 563–71.
E.M. Levin, C.R. Robbins, and H.F. McMurdie: Phase Diagrams for Ceramists, 2nd ed., The American Ceramic Society Inc., Columbus, OH, 1969, p. 228.
F. Matsuno: T. Iron Steel I. Japan, 1979, vol. 19, pp. 595–604.
F. Matsuno and T. Harada: T. Iron Steel I. Japan, 1981, vol. 21, pp. 318–25.
M.I. Pownceby and J.M.F. Clout: T. I. Min. Metall. C, 2000, vol. 109, pp. 36–48.
N.V.Y. Scarlett, M.I. Pownceby, I.C. Madsen, and A. Christensen: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 929–36.
N.V.Y. Scarlett, I.C. Madsen, M.I. Pownceby, and A. Christensen: J. Appl. Crystallogr., 2004, vol. 37, pp. 362–68.
N.A.S. Webster, M.I. Pownceby, I.C. Madsen, N.V.Y. Scarlett, L. Lu, and J.R. Manuel: Australas. I. Min. Met., 2011, no. 6, pp. 537–43.
K. Wallwork, B. Kennedy, and D. Wang: AIP Conf. Proc., 2007, vol. 879, pp. 879–82.
B. Schmitt, C. Brönnimann, E.F. Eikenberry, F. Gozzo, C. Hörmann, R. Horisberger, and B. Patterson: Nucl. Instrum. Methods A, 2003, vol. 501, pp. 267–72.
M.R. Rowles: Powder Diff., 2010, vol. 25, pp. 297–301.
H.M. Rietveld: J. Appl. Crystallogr., 1967, vol. 2, pp. 65–71.
R.B. Von Dreele: Powder Diffraction: Theory and Practice, R.E. Dinnebier and S.J.L. Billinge, eds., Royal Society of Chemistry, Cambridge, U.K., 2008, pp. 266–81.
Bruker: 2009, TOPAS Version 4.2, Bruker AXS Inc., Madison, WI.
D. Taylor: Brit. Ceram. Trans. J., 1984, vol. 83, pp. 92–98.
B.K. Gan, I.C. Madsen, and J.G. Hockridge: J. Appl. Crystallogr., 2009, vol. 42, pp. 697–705.
K. Kihara: Eur. J. Mineral., 1990, vol. 2, pp. 63–77.
S.A. Markgraf and R.J. Reeder, Am. Mineral., 1985, vol. 70, pp. 590–600.
R. Blake, R. Hessevick, T. Zoltai, and L. Finger: Am. Mineral., 1966, vol. 51, pp. 123–29.
H. Schulz and V. Tscherry: Acta Crystallogr. B Struct., 1972, vol. 28, pp. 2168–73.
I. Oftedal: Z. Phys. Chem., 1927, vol. 128, pp. 135–58.
P. Berastegui, S.-G. Eriksson, and S. Hull: Mater. Res. Bull., 1999, vol. 34, pp. 303–14.
B.F. Decker and J.S. Kasper: Acta Crystallogr., 1957, vol. 10, pp. 332–37.
R.R. Dayal and F.P. Glasser: Sci. Ceram., 1967, vol. 3, pp. 191–214.
J.S. Huebner: Research Techniques for High Temperature and High Pressure, G.C. Ulmer, ed., Springer, New York, NY, 1971, pp. 123–77.
G.J. Redhammer, G. Tippelt, G. Roth, and G. Amthauer: Am. Mineral., 2004, vol. 89, pp. 405–20.
M.I. Pownceby and J.M.F. Clout: T. I. Min. Metall. C, 2003, vol. 112, pp. 44–51.
E.J. Bagnall: Proc. 2nd Int. Symp. Agglomeration, 1977, Atlanta, GA, pp. 587–603.
P.R. Dawson, J. Ostwald, and K.M. Hayes: Proc. Australas. Inst. Min. Metall., 1984, no. 289, pp. 163–69.
M.S. Model, T.Y. Malysheva, V.Y. Lyadova, N.V. Chugunova, E.V. Vlasova, and G.K. Astakhova: Russ. Metall., 1987, vol. 2, pp. 1–7.
C.E. Loo, R.P. Williams, and L.T. Matthews: T. I. Min. Metall. C, 1992, vol. 101, pp. 7–16.
E. Da Costa, J.P. Coheur, B. Vanderheyden, and R. Munnix: ISIJ Int., 1995, vol. 35, pp. 138–47.
R.D. Shannon: Acta Crystallogr. A Crys., 1976, vol. 32, pp. 751–67.
A.R. West: Basic Solid State Chemistry, 2nd ed., Wiley, Chichester, U.K., 1999, p. 108.
Acknowledgments
The Australian Nuclear Science and Technology Organization (ANSTO) are acknowledged for their financial support of this research. This research was partially undertaken on the powder diffraction beamline (10BM1) at the Australian Synchrotron, Victoria, Australia, under beamtime awards AS093/PD1639 and AS113/PD4160. The authors wish to thank: James Manuel, Liming Liu, Nicola Scarlett, Mark Styles, Jean-Pierre Veder, Caroline Johnson, Barry Halstead, and Helen Brand (all CSIRO Process Science and Engineering), Kia Wallwork (Australian Synchrotron) for assistance with synchrotron data collection; Matthew Glenn and Aaron Torpy (CSIRO Process Science and Engineering) for assistance with scanning electron microscopy; and Nick Wilson and Colin McRae (CSIRO Process Science and Engineering) for assistance with electron microprobe analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 12, 2012.
Rights and permissions
About this article
Cite this article
Webster, N.A.S., Pownceby, M.I., Madsen, I.C. et al. Silico-ferrite of Calcium and Aluminum (SFCA) Iron Ore Sinter Bonding Phases: New Insights into Their Formation During Heating and Cooling. Metall Mater Trans B 43, 1344–1357 (2012). https://doi.org/10.1007/s11663-012-9740-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9740-5