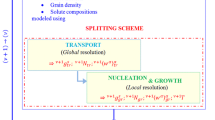
Abstract
Although a significant amount of work has already been devoted to the prediction of macrosegregation in steel ingots, most models considered the solid phase as fixed. As a result, it was not possible to correctly predict the macrosegregation in the center of the product. It is generally suspected that the motion of the equiaxed grains is responsible for this macrosegregation. A multiphase and multiscale model that describes the evolution of the morphology of the equiaxed crystals and their motion is presented. The model was used to simulate the solidification of a 3.3-ton steel ingot. Computations that take into account the motion of dendritic and globular grains and computations with a fixed solid phase were performed, and the solidification and macrosegregation formation due to the grain motion and flow of interdendritic liquid were analyzed. The predicted macrosegregation patterns are compared to the experimental results. Most important, it is demonstrated that it is essential to consider the grain morphology, in order to properly model the influence of grain motion on macrosegregation. Further, due to increased computing power, the presented computations could be performed using finer computational grids than was possible in previous studies; this made possible the prediction of mesosegregations, notably A segregates.











Similar content being viewed by others
Abbreviations
- C i :
-
concentration of element i
- C d :
-
drag coefficient
- D :
-
diffusion coefficient
- d g :
-
equivalent grain diameter
- d s :
-
equivalent solid phase diameter
- \( \vec{g}\) :
-
gravity acceleration
- g block :
-
packing grain-volume fraction
- g env :
-
grain (dendrite envelope) volume fraction
- g l :
-
liquid volume fraction
- g s :
-
solid volume fraction
- g c s :
-
grain impingement limit
- g si :
-
internal solid fraction
- h :
-
specific enthalpy
- k :
-
thermal conductivity
- K :
-
permeability
- k i p :
-
partition coefficient for element i
- l l :
-
primary dendrite arm length
- m i L :
-
liquidus slope for element i
- N :
-
grain density (number of grains per unit volume)
- P :
-
pressure
- Re:
-
Reynolds number
- S env :
-
envelope surface area density
- S V :
-
solid-liquid interfacial area density
- Sc:
-
Schmidt number
- SDAS:
-
secondary dendrite arm spacing
- t :
-
time
- T :
-
temperature
- T f :
-
melting temperature of pure iron
- \( \vec{v}\) :
-
velocity
- V 0 :
-
initial grain volume at nucleation
- V env :
-
initial grain volume at nucleation
- V tip :
-
dendrite tip velocity
- β i C :
-
solutal expansion coefficient for element i
- β T :
-
thermal expansion coefficient
- Γ env :
-
grain (dendrite envelope) growth rate
- Γ s :
-
solid growth rate
- δ l :
-
solute diffusion length in the liquid
- δ s :
-
solute diffusion length in the solid
- μ l :
-
liquid viscosity
- ρ :
-
density
- ρ g :
-
density in the buoyancy term
- Φ s :
-
solid mass generation due to nucleation
- env :
-
grain envelope
- l :
-
liquid phase
- s :
-
solid phase
- *:
-
equilibrium at the solid-liquid interface
References
A. Hultgren: Scand. J. Metall., 1973, vol. 2, pp. 217–27
M.C. Flemings: Scand. J. Metall., 1976, vol. 5, pp. 1–15
R. Mehrabian, M. Keane, M.C. Flemings: Metall. Trans., 1970, vol. 1, pp. 1209–20
Y.-K. Chuang, K. Schwerdtfeger: Arch. Eisenhüttenwes, 1975, vol. 46, pp. 303–10
A. Olsson, R. West, H. Fredriksson: Scand. J. Metall., 1986, vol. 15, pp. 104–12
W.D. Bennon, F.P. Incropera: Int. J. Heat Mass Transfer, 1987, vol. 30, pp. 2161–70
S. Ganesan, D.R. Poirier: Metall. Trans. B, 1990, vol. 21B, pp. 173–81
D.R. Poirier, P.J. Nandapurkar, S. Ganesan: Metall. Trans. B, 1991, vol. 22B, pp. 889–900
J. Ni, C. Beckermann: Metall. Trans. B, 1991, vol. 22B, pp. 349–61
I. Ohnaka: in State of the Art of Computer Simulation of Casting and Solidification Processes, H. Fredriksson, ed., Les éditions de physique, Les Ulis, France, 1986, pp. 211–23
F. Roch, H. Combeau, J, Chevrier, and G. Lesoult: in Modeling of Casting, Welding and Advanced Solidification Processes V, M. Rappaz, M.R. Özgü, and K.W. Mahin, eds., TMS, Warrendale, PA, 1991, pp. 789–96
F. Roch, H. Combeau, I. Poitrault, J. Chevrier, G. Lesoult: 6th Int. Iron and Steel Congr., ISIJ, Nagoya, Japan, 1990, pp. 665–72
I. Vannier, H. Combeau, and G. Lesoult: Numiform ‘92, 4th International Conference on Numerical Methods in Industrial Forming Processes, J.-L. Chenot, R.D. Wood, and O.C. Zienkiewicz, eds., Balkema, Rotterdam, 1992, pp. 835–40
I. Vannier, H. Combeau, G. Lesoult: Mater. Sci. Eng., A, 1993, vol. 173, pp. 317–21
J. Common, J. Delorme, P. Bastien: Rev. Metall., 1973, vol. 70(4), pp. 251–58
J.P. Gu, C. Beckermann: Metall. Mater. Trans. A, 1999, vol. 30A, pp. 1357–66
C.Y. Wang, C. Beckermann: Metall. Mater. Trans. A, 1996, vol. 27A, pp. 2754–64
B. Appolaire, V. Albert, H. Combeau, G. Lesoult: Acta Mater., 1998, vol. 46, pp. 5851–62
B. Appolaire, V. Albert, H. Combeau, G. Lesoult: ISIJ, 1999, vol. 39, pp. 263–70
A. Badillo, D. Ceynar, C. Beckermann: J. Cryst. Growth, 2007, vol. 309, pp. 197–215
A. Badillo, D. Ceynar, C. Beckermann: J. Cryst. Growth, 2007, vol. 309, pp. 216–24
B. Appolaire and H. Combeau: Int. Symp. Liquid Metal Processing and Casting, Ecole des Mines de Nancy, Nancy, France, 2003, pp. 233–39
B. Rabia: Doctoral Thesis, Institut National Polytechnique de Lorraine, Ecole des Mines de Nancy, Nancy, France, 2004
C. Camperola: Ecole des Mines d’Albi, Albi, France, internal note, 2003
I. Vannier: Doctoral Thesis, Institut National Polytechnique de Lorraine, Ecole des Mines de Nancy, Nancy, France, 1995
N. Ahmad, H. Combeau, J.-L. Desbiolles, T. Jalanti, G. Lesoult, J. Rappaz, M. Rappaz, C. Stomp: Metall. Mater. Trans. A, 1998, vol. 29A, pp. 617–30
J. Ni and C. Beckermann: J. Mater. Process. Manuf. Sci., 1993, vol. 2, pp. 217–31
S. Charmond: Technical Report, Ecole Nationale Supérieure de Chimie de Clermont-Ferrand, Clermont-Ferrand, France, 2005
S. Charmond: Master’s Thesis, Ecole Nationale Supérieure de Chimie de Clermont-Ferrand, Clermont-Ferrand, France, 2006
B. Appolaire, H. Combeau, G. Lesoult: Mater. Sci. Eng., A, 2008, vol. 487, pp. 33–45
W. Kurz, B. Giovanola, R. Trivedi: Acta Metall., 1986, vol. 34, pp. 823–30
M.C. Schneider, C. Beckermann: Metall. Mater. Trans. A, 1995, vol. 26A, pp. 2373–87
M. Založnik and B. Šarler: in Modeling of Casting, Welding and Advanced Solidification Processes XI, C.-A. Gandin and M. Bellet, eds., TMS, Warrendale, PA, 2006, pp. 243–50
C. Beckermann: Int. Mater. Rev., 2002, vol. 47, pp. 243–61
Acknowledgments
This work was supported by the research program OSC, which was co-financed by the French research ministry and eight industrial partners (Aubert & Duval, Ascometal, CTIF, Erasteel, Fonderie de l’Atlantique, Industeel, PSA, SCC, and Transvalor), and in part by a consortium of ArcelorMittal, Ascometal, Aubert & Duval, Erasteel, and Rio Tinto Alcan.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is based on a presentation given at the International Symposium on Liquid Metal Processing and Casting (LMPC 2007), which occurred in September 2007 in Nancy, France.
Appendix
Appendix
Adding the average solute mass balance in the solid and liquid phases (Eqs. [6] and [7]) and accounting for relation [21], one gets
with \( \rho _m C_m = \rho _s g_s C_s + \rho _l g_l C_l \).
By applying mass conservation (Eq. [3]) and assuming equal and constant densities of the solid and liquid phases, Eq. [A1] becomes
Assuming in addition, the local thermodynamic equilibrium in the entire liquid and solid phases (lever rule), the partial time derivative of the average local solute mass fraction can then be expressed as
For a negative liquidus slope m L , the first term of Eq. [A3] means that any motion of the solid and liquid phases, such that the velocity component parallel to the thermal gradient is oriented in the same direction as the thermal gradient, will induce an increase in the average solute mass fraction. At the reverse, if the parallel velocity component is in the direction opposite to the thermal gradient, a decrease in the average solute mass fraction will be observed. The second (grain transport) term quantifies the contribution of the transport of solid grains (purely passive transport, i.e., without phase change) on the average solid mass fraction. Note that this equation allows a direct interpretation of the circulation of the solid and liquid phases on the variation in the local solute content; in the more general case, however, in which shrinkage is accounted for, it is possible to derive only a relation between the relative motion of the phases and the partial derivative of the solute mass fraction of the liquid vs the volume fraction of the liquid.[2]
Rights and permissions
About this article
Cite this article
Combeau, H., Založnik, M., Hans, S. et al. Prediction of Macrosegregation in Steel Ingots: Influence of the Motion and the Morphology of Equiaxed Grains. Metall Mater Trans B 40, 289–304 (2009). https://doi.org/10.1007/s11663-008-9178-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-008-9178-y