Abstract
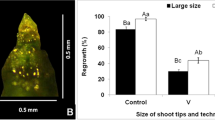
Here, we report an efficient and widely applicable method for cryopreservation of Malus shoot tips by encapsulation–dehydration using adventitious shoots. Shoots were induced from leaf segments cultured on a shoot induction medium containing 2–3 mg L−1 thidiazuron, depending on genotype, and 0.5 mg L−1 indole-3-butyric acid. Shoot tips (3 mm in length) containing six leaf primordia excised from 11-wk-old adventitious shoots were encapsulated and precultured with 0.5 M sucrose for 5 d, followed by air-drying for 6 h prior to direct immersion in liquid nitrogen. With our protocol, we obtained a mean organogenesis rate of 100%, a mean of 4.5 adventitious shoots per explant (leaf segment), and a mean shoot recovery of 57.0% from cryopreserved shoot tips in four Malus species. Inter-simple sequence repeat (ISSR) analysis did not reveal any polymorphic bands in regenerants recovered from either leaf segments or cryopreserved shoot tips of ‘Gala’. To the best of our knowledge, this is the first report on cryopreservation of Malus shoot tips using adventitious shoots derived from leaf segments and is the most widely applicable protocol so far reported for cryopreservation of Malus. Establishment of this protocol provides an alternative means for cryopreservation of Malus.






Similar content being viewed by others
References
Aldwinckle H, Malnoy M (2009) Plant regeneration and transformation in the Rosaceae. Transgenic Plant J 3:1–39
Aronen T, Krajnakova J, Häggman H, Ryynänen L (1999) Genetic fidelity of cryopreserved embryogenic cultures of open-pollinated Abies cephalonica. Plant Sci 142:163–172
Benson EE (2008) Cryopreservation of phytodiversity: a critical appraisal of theory & practice. Crit Rev Plant Sci 27:141–219
Brischia R, Piccioni E, Standardi A (2002) Micropropagation and synthetic seed in M.26 apple rootstock (II): a new protocol for production of encapsulated differentiating propagules. Plant Cell Tissue Organ Cult 68:137–141
Burritt DJ (2008) Efficient cryopreservation of adventitious shoots of Begonia × erythrophylla using encapsulation–dehydration requires pretreatment with both ABA and proline. Plant Cell Tissue Organ Cult 95:209–215
Caboni E, Lauri P, Damiano C, D’Angeli S (2000) Somaclonal variation induced by adventitious shoot regeneration in pear and apple. Acta Horticult 530:195–201
Condello E, Caboni E, Andre E, Piette B, Druart P, Swennen R, Panis B (2011) Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 32:175–185
Dereuddre J, Fabre J, Bassaglia C (1988) Resistance to freezing in liquid nitrogen of carnation (Dianthus caryophyllus L. var. Eolo) apical and axillary shoot tips excised from different aged in vitro plantlets. Plant Cell Rep 7:170–173
Devarumath RM, Nandy S, Rani V, Marimuthu S, Muraleedharan N, Raina SN (2002) RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. Assamica (Assam-India type). Plant Cell Rep 21:166–173
Dobránszki J, Hudák I, Magyar-Tábori K, Galli Z, Kiss E, Jámbor-Benczúr E (2006) How can different cytokinins influence the process of shoot regeneration from apple leaves in ‘Royal Gala’ and ‘M.26’. Acta Horticult 725:191–196
Dobránszki J, Hudak I, Magyar-Tabori K, Jambor-Benczur E, Galli Z, Kiss E (2004) Effects of different cytokinins on the shoot regeneration from apple leaves of ‘Royal Gala’ and ‘M.26’. Int J Hortic Sci 101:69–75
Dobránszki J, Teixeira da Silva JA (2010) Micropropagation of apple—a review. Biotechnol Adv 28:462–488
Engelmann F, Callow JA, Ford-Lloyd BV, Newbury HJ (1997) In vitro conservation methods. In: Callow JA, Ford-Lloyd BV, Newbury HJ (eds) Biotechnology and plant genetic resources: conservation and use. Biotechnology in agriculture series, vol 19. CAB International, Oxon, pp 119–161
Famiani F, Ferradini N, Staffolani P, Standari A (1994) Effect of leaf excision time and age, BA concentration and dark treatments on in vitro shoot regeneration of M.26 apple rootstock. J Hortic Sci 69:679–685
Fasolo F, Zimmerman RH, Fordham I (1989) Adventitious shoot formation on excised leaves of in vitro grown shoots of apple cultivars. Plant Cell Tissue Organ Cult 16:75–87
Feng CH, Cui ZH, Li BQ, Chen L, Ma YL, Zhao YH, Wang QC (2013) Duration of sucrose preculture is critical for shoot regrowth of in vitro-grown apple shoot-tips cryopreserved by encapsulation-dehydration. Plant Cell Tissue Organ Cult 112:369–378
Gamage N, Nakanishi T (2000) In vitro shoot regeneration from leaf tissue of apple (cultivar ‘Orine’): high shoot proliferation using carry over effect of TDZ. Acta Horticult 520:291–298
Gupta R, Modgil M, Chakrabarti SK (2009) Assessment of genetic fidelity of micropropagated apple rootstock plants, EMLA 111, using RAPD markers. Ind J Exp Bot 47:925–928
Halmagyi A, Deliu C, Coste A (2005) Plant regrowth from potato shoot tips cryopreserved by a combined vitrification-droplet method. CryoLetters 26:313–322
Halmagyi A, Deliu C, Isac V (2010) Cryopreservation of Malus cultivars: comparison of two droplet protocols. Sci Hortic 124:387–392
Hao YJ, Liu QL, Deng XX (2001) Effect of cryopreservation on apple genetic resources at morphological, chromosomal, and molecular levels. Cryobiology 43:46–53
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. CryoLetters 25:3–22
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (2002) Sucrose incubation increases freezing tolerance of asparagus (Asparagus officinalis L.) embryogenic cell suspensions. CryoLetters 23:103–112
Korban SS, O’Connor PA, Elobeidy A (1992) Effect of thidiazuron, naphthalene acetic acid, dark incubation and genotype on shoot organogenesis of Malus leaves. J Hortic Sci 67:341–349
Kuo CC, Lineberger RD (1985) Survival of in vitro cultured tissue of ‘Jonathan’ apples exposed to −196°C. HortSci 20:764–767
Kushnarenko SV, Romadanova NV, Reed BM (2009) Cold acclimation improves regrowth of cryopreserved apple shoot tips. CryoLetters 30:47–54
Li YN (1999) Progress in research on the origin and evolution of the genus Malus in the world. J Fruit Sci 16:8–19
Liu JR, Sink KC, Dennis FG (1983) Plant regeneration from apple seedling explants and callus cultures. Plant Cell Tissue Organ Cult 2:293–304
Liu YG, Liu LX, Wang L, Gao AY (2008) Determination of genetic stability in surviving apple shoots following cryopreservation by vitrification. CryoLetters 29:7–14
Liu YG, Wang XY, Liu LX (2004) Analysis of genetic variation in surviving apple shoots following cryopreservation by vitrification. Plant Sci 166:677–685
Magyar-Tábori K, Dobránszki J, Teixeira da Silva JA, Bulley SM, Hudák I (2010) The role of cytokinins in shoot organogenesis in apple. Plant Cell Tissue Organ Cult 101:251–267
Matsumoto T, Sakai A, Yamada K (1995) Cryopreservation of in vitro-grown apical meristems of lily by vitrification. Plant Cell Tissue Organ Cult 41:237–241
Mitić N, Stanišić M, Milojević J, Tubić L, Ćosić T, Nikolić R, Ninković S, Miletić R (2012) Optimization of in vitro regeneration from leaf explants of apple cultivars ‘Golden Delicious’ and ‘Melrose’. HortSci 47:1117–1122
Modgil M, Mahajan K, Chakrabarti SK, Sharma DR, Sobti RC (2005) Molecular analysis of genetic stability in micropropagated apple rootstock MM106. Sci Hortic 104:151–160
Montecelli S, Gentile A, Damino C (2000) In vitro shoot regeneration of apple cultivar Gala. Acta Horticult 530:219–223
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Niino T, Sakai A (1992) Cryopreservation of alginate-coated in vitro-grown shoot tips of apple, pear and mulberry. Plant Sci 87:199–206
Niino T, Sakai A, Yakuwa H, Nojiri K (1992) Cryopreservation of in vitro-grown shoot tips of apple and pear by vitrification. Plant Cell Tissue Organ Cult 28:261–266
Pathak H, Dhawan V (2010) Molecular analysis of micropropagated apple rootstock MM111 using ISSR markers for ascertaining clonal fidelity. Acta Horticult 865:73–80
Pathak H, Dhawan V (2012) ISSR assay for ascertaining genetic fidelity of micropropagated plants of apple rootstock Merton 793. In Vitro Cell Dev Biol Plant 48:137–143
Paul H, Daigny G, Sangwan-Norreel BS (2000) Cryopreservation of apple (Malus × domestica Borkh.) shoot tips following encapsulation-dehydration or encapsulation-vitrification. Plant Cell Rep 19:768–774
Pinker I, Halmagyi A, Olbricht K (2009) Effects of sucrose preculture on cryopreservation by droplet-vitrification of strawberry cultivars and morphological stability of cryopreserved plants. CryoLetters 30:202–211
Rani V, Raina SN (1998) Genetic analysis of enhanced axillary branching derived Eucalyptus tereticornis and E. camaldulensis plants. Plant Cell Rep 17:236–242
Ray T, Dutta I, Saha P, Das S, Roy SC (2006) Genetic stability of three economically important micropropagated banana (Musa spp.) cultivars of lower Indo-Gangetic plains, as assessed by RAPD and ISSR markers. Plant Cell Tissue Organ Cult 85:11–21
Sarwar M, Skirvin RM (1997) Effect of thidiazuron and 6-benzylaminopurine on adventitious shoot regeneration from leaves of three strains of ‘McIntosh’ apple (Malus × domestica Borkh.) in vitro. Sci Hortic 68:95–100
Sriskandarajah S, Skirvin RM, Abu-Qaoud H, Korban SS (1990) Factors involved in shoot elongation and growth of adventitious and axillary shoots of three apple scion cultivars in vitro. J Hortic Sci 65:113–121
Theiler-Hedtrich C, Theiler-Hedtrich R (1990) Influence of TDZ and BA on adventitious shoot regeneration from apple leaves. Acta Horticult 280:196–199
Towill LE, Forshline PL, Walters C, Waddell JW, Laufmann J (2004) Cryopreservation of Malus germplasm using a winter vegetative bud method: results from 1915 accessions. CryoLetters 25:323–334
Venkatachalam L, Sreedhar RV, Bhagyalakshmi N (2007) Micropropagation in banana using high levels of cytokinins does not involve any genetic changes as revealed by RAPD and ISSR markers. Plant Growth Regul 51:193–205
Viršcek-Marn M, Javornik B, Štampar F, Bohanec B (1998) Assessment of genetic variation among regenerants from in vitro apple leaves using molecular markers. Acta Horticult 484:299–303
Wang QC, Laamanen J, Uosukainen M, Valkonen JP (2005) Cryopreservation of in vitro-grown shoot tips of raspberry (Rubus idaeus L.) by encapsulation–vitrification and encapsulation–dehydration. Plant Cell Rep 24:280–288
Wang QC, Perl A (2006) Cryopreservation in floricultural plants. In: da Silva T (ed) Floriculture, ornamental and plant biotechnology. Global Science Books, London, pp 523–539
Wang QC, Valkonen JPT (2008) Eradication of two synergistically interacting viruses from sweetpotato using shoot tip culture and cryotherapy of shoot tips. J Virol Meth 154:135–145
Welander M (1988) Plant regeneration from leaf and stem segments of shoots raised in vitro from mature apple trees. J Plant Physiol 132:738–744
Wu YJ, Engelmann F, Zhao YH, Zhou MD, Chen SY (1999) Cryopreservation of apple shoot tips: importance of cryopreservation technique and of conditioning of donor plants. CryoLetters 20:121–130
Yin ZF, Zhao B, Bi WL, Chen L, Wang QC (2013) Direct shoot regeneration from basal leaf segments of Lilium and assessment of genetic stability in regenerants by ISSR and AFLP markers. In Vitro Cell Dev Biol Plant 49:333–342
Yoon JW, Kim HH, Ko HC, Hwang HS, Hong ES, Cho EG, Engelmann F (2006) Cryopreservation of cultivated and wild potato varieties by droplet vitrification: effect of subculture of mother-plants and of preculture of shoot tips. CryoLetters 27:211–222
Zhao YH, Wu YJ, Engelmann F, Zhou MD, Chen SY (1999) Cryopreservation of apple in vitro shoot tips by the droplet freezing method. CryoLetters 20:109–112
Acknowledgments
The authors acknowledge financial support from Northwest A&F University (Z222020904) and from the Department of Fruit Industry of Shaanxi Province (K336021105).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Barbara Reed
Bai-Quan Li and Chao-Hong Feng contributed equally to the present study
Rights and permissions
About this article
Cite this article
Li, BQ., Feng, CH., Hu, LY. et al. Shoot regeneration and cryopreservation of shoot tips of apple (Malus) by encapsulation–dehydration. In Vitro Cell.Dev.Biol.-Plant 50, 357–368 (2014). https://doi.org/10.1007/s11627-014-9616-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-014-9616-2