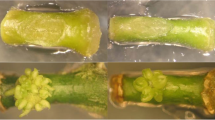
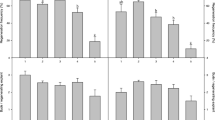
Abstract
Effective regeneration in vitro is a necessary precondition for the implementation of different biotechnological approaches in plant breeding. Numerous studies have reported on regeneration from apple somatic tissues, and organogenesis has been proved to be influenced by several factors including mother shoots (genotype, size, type, and age of explant), in vitro conditions (dark period, light intensity, and quality), and others (wounding, orientation of leaf explants). However, one of the most important factors before and during the regeneration process is the type and concentration of cytokinin applied. Thidiazuron and benzyladenine are the most frequently used cytokinins in the regeneration systems, but their efficiency depends on genotype and other factors. Other cytokinins (e.g., zeatin and kinetin) have also been tested in several experiments and they were found in general to be less active. The organogenic ability of explants can also be increased by a properly selected cytokinin pre-treatment. Cytokinins applied in the pre-treatments can influence the leaf structure, which in turn can alter the regeneration capacity of the leaf explant. Interactions between factors of pre-treatments (hormones, light, and culture conditions) and factors of the regeneration phase should be considered. This review brings into focus the role of different cytokinins during in vitro shoot development, discussing their effects on the histology of leaves developed in vitro, and how this affects the subsequent regeneration process.




Similar content being viewed by others
References
Barbieri C, Morini S (1987) In vitro regeneration from somatic tissues and seed explants of apple. Adv Hortic Sci 1:8–10
Barciszewski J, Rattan SIS, Siboska G, Clark BFC (1999) Kinetin—45 years on. Plant Sci 148:37–45
Bartish IV, Korkhovoi VI (1997) The composition of nutrient medium and the efficiency of shoot induction in vitro from apple leaf explants. Russ J Plant Physiol 44:381–385
Belaizi M, Paul H, Sangwan RS, Sangwan-Norreel BS (1991) Direct organogenesis from internodal segments of in vitro grown shoots of apple cv. Golden delicious. Plant Cell Rep 9:471–474
Benková E, Witters E, van Dongen W, Kolář J, Motyka V, Brzobohatý B, van Onckelen HA, Macháčková I (1999) Cytokinins in tobacco and wheat chloroplasts. Occurrence and changes due to light/dark treatment. Plant Physiol 121:245–252
Bulley SMW, James DJ (2004) Regeneration and genetic transformation of apple (Malus spp.). In: Curtis IS (ed) Transgenic crops of the world: essential protocols, chapter 15. Springer, Dordrecht
Bulley SM, Malnoy M, Atkinson RG, Aldwinckle HS (2007) Transformed apples: Traits of significance to growers and consumers. Transgen Plant J 1:267–279
Caboni E, Tonelli MG (1999) Effect of 1, 2-benzisoxazole-3-acetic acid on adventitious shoot regeneration and in vitro rooting in apple. Plant Cell Rep 18:985–988
Caboni E, Tonelli M, Falasca G, Damiano C (1996) Factors affecting adventitious shoot regeneration in vitro in the apple rootstock ‘Jork 9’. Adv Hortic Sci 10:146–150
Caboni E, Lauri P, D’Angeli S (2000) In vitro plant regeneration from callus of shoot apices in apple shoot culture. Plant Cell Rep 19:755–760
Caboni E, D’Angeli S, Chiappetta A, Innocenti AM, Van Onckelen H, Damiano C (2002) Adventitous shoot regeneration from vegetative shoot apices in pear and putative role of cytokinin accumulation in the morphogenetic process. Plant Cell Tissue Org Cult 70:199–206
Camargo JT, Fortes GR, Silva JB, Centellas AQ, Oliveira MdeF (1998) Evaluation of BAP concentrations for callogenesis/organogenesis in apical and basal internodes of the apple rootstock cv. Marubakaido (Malus prunifolia). Agropecu Clima Temperado 1:129–136
Chen JX, Ziv M (2005) The effects of storage condition on starch metabolism and regeneration potentials of twin-scales and inflorescence stem explants of Narcissus tazetta. In Vitro Cell Dev Biol Plant 41:816–821
D’Angeli S, Lauri P, Dewitte W, Van Onckelen H, Caboni E (2001) Factors affecting in vitro shoot formation from vegetative shoot apices of apple and relationship between organogenic response and cytokinin localisation. Plant Biosyst 135:95–100
De Bondt A, Eggermont K, Druart P, De Vil M, Goderis I, Vanderleyden J, Broekaert WF (1994) Agrobacterium-mediated transformation of apple (Malus domestica Borkh.): an assessment of factors affecting gene transfer efficiency during early transformation steps. Plant Cell Rep 13:587–593
De Bondt A, Eggermont K, Penninckx I, Goderis I, Broekaert WF (1996) Agrobacterium-mediated transformation of apple (Malus x domestica Borkh.): an assessment of factors affecting regeneration of transgenic plants. Plant Cell Rep 15:549–554
Dobránszki J, Magyar-Tábori K, Jámbor-Benczúr E, Kiss E, Bubán T (2001) Post-effects of meta-topolin on morphogenic activity of in vitro leaves of apple ‘Royal Gala’. COST 843, WG1: Developmental Biology of Regeneration, 2nd Meeting, 18–20 Oct. 2001, Rome, Italy, pp 30–31
Dobránszki J, Magyar-Tábori K, Jámbor-Benczúr E, Kiss E, Lazányi J, Bubán T (2002) Effect of conditioning apple shoots with meta-topolin on the morphogenic activity of in vitro leaves. Acta Agron Hung 50:117–126
Dobránszki J, Hudák I, Magyar-Tábori K, Jámbor-Benczúr E, Galli ZS, Kiss E (2004) Effects of different cytokinins on the shoot regeneration from apple leaves of ‘Royal Gala’ and ‘M.26’. Int J Hortic Sci 10:69–75
Dobránszki J, Jámbor-Benczúr E, Reményi ML, Magyar-Tábori K, Hudák I, Kiss E, Galli ZS (2005) Effects of aromatic cytokinins on structural characteristics of leaves and their post-effects on subsequent shoot regeneration from in vitro apple leaves of ‘Royal Gala’. Int J Hortic Sci 11:41–46
Dobránszki J, Hudák I, Magyar-Tábori K, Jámbor-Benczúr E, Galli ZS, Kiss E (2006) How can different cytokinins influence the process of shoot regeneration from apple leaves in ‘Royal Gala’ and ‘M26’. Acta Hortic 725:191–196
Dufour M (1990) Improving yield of adventitious shoots in apple. Acta Hortic 280:51–60
Ďurkovič J, Mišalová A (2008) Micropropagation of temperate noble hardwoods: An overview. Funct Plant Sci Biotechnol 2:1–19
Elobeidy A, Korban SS (1988) The effect of thidiazuron on shoot regeneration from apple leaf discs. Hortic Sci 23:755
Erig AC, Schuch MW, Silva LC (2004) Effect of cytokinins and of the origin of the explant in the in vitro organogenesis of shoots of apple tree (Malus domestica Borkh.) cv. Galaxy. Rev Cient Rural Intec, Bage, Brazil 9:113–120
Famiani F, Ferradini N, Staffolani P, Standardi A (1994) Effect of leaf excision time and age, BA concentration and dark treatments on in vitro shoot regeneration of M.26 apple rootstock. J Hortic Sci 69:679–685
FAO (2009) http://faostat.fao.org/site/567/Desktopdefault.aspx?PageID=567#ancor
Fasolo FMF, Predieri S (1990) Cultivar dependent responses to regeneration from leaves in apple. Acta Hortic 280:61–68
Fasolo FMF, Zimmerman RH, Fordham I (1989) Adventitious shoot formation on excised leaves of in vitro grown shoots of apple cultivars. Plant Cell Tissue Org Cult 16:75–87
Ferradini N, Famiani F, Proietti P, Stanica F (1996) Influence of growth regulators and light on in vitro shoot regeneration in M.26 apple rootstock. J Hortic Sci 71:859–865
Ferreira FJ, Kieber JJ (2005) Cytokinin signalling. Current Opin Plant Biol 8:518–525
Fortes AM, Pais MS (2000) Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. Am J Bot 87:971–979
Gahan PB, George EF (2008) Adventitious regeneration. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 355–401
Gamage N, Nakanishi T (2000) In vitro shoot regeneration from leaf tissue of apple (cultivar “Orine”): high shoot proliferation using carry over effect of TDZ. Acta Hortic 520:291–300
Gercheva P, Andinova T, Ivanova K (2000) Plant regeneration from leaf tissues of apple cultivars Granny Smith, Morspur Golden and Starkrimson. Bulg J Agric Sci 6:637–643
Gulewicz K, Tykarska T, Wysocki W, Augustynowicz J, Zurowska K, Kuraś M (2004) Developmental and ultrastructural effect of Uncaria tomentosa (Willd.) DC extract on the paprika Capsicum annuum L. Ind Crop Prod 19:59–67
Hanke V, Rohde A, Grafe C (1991) Untersuchungen zur Regeneration an somatischen Gewebe in vitro. I. Zur Adventivsprossbildung an Blattexplantaten bei Apfel (Malus domestica Borkh). Studies on regeneration from somatic tissue in vitro. I. Adventitious shoot formation on leaf explants in apple (Malus domestica Borkh). Gartenbauwiss 56:214–220
Hazarika BN (2006) Morpho-physiological disorders in in vitro culture of plants. Sci Hortic 108:105–120
Heyl A, Schmülling T (2003) Cytokinin signal perception and transduction. Curr Opin Plant Biol 6:480–488
Howell SH, Lall S, Che P (2003) Cytokinins and shoot development. Trends Plant Sci 8:453–459
Hudák I, Dobránszki J, Magyar-Tábori K, Jámbor-Benczúr E, Galli Zs, Kiss E (2003) Effects of benzyladenine analogues on the organogenetic ability of apple leaves. Book of abstracts, 5th international symposium in the series recent advances in plant biotechnology. Plant Biotechnology: Progress and Developments. Stará Lesná, September 7–13, High Tatras, Slovak Republic, p 22
Hudák I, Dobránszki J, Magyar-Tábori K, Jámbor-Benczúr E, Galli Zs, Kiss E (2005) Effects of aromatic cytokinins on the organogenesis in apple. Book of abstracts COST 843 final conference COST 843 and COST 851 joint meeting, June 28–July 3, 2005, Stará Lesná, Slovakia, p 82–84
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Org Cult 33:105–119
Jámbor-Benczúr E, Kissimon J, Fábián M, Mészáros A, Sinkó Z, Gazdag GY, Nagy T (2001) In vitro rooting and anatomical study of leaves and roots of in vitro and ex vitro plants of Prunus x davidopersica ‘Piroska’. Int J Hortic Sci 7:1–5
James DJ, Dandekar AM (1991) Regeneration and transformation of apple (Malus pumila Mill.). Plant Tissue Cult Manage B8:1–18
James DJ, Passey AJ, Rugini E (1988) Factors affecting high frequency plant regeneration from apple leaf tissues cultured in vitro. J Plant Physiol 132:148–154
Jones OP, Hopgood ME, O’Farrel D (1977) Propagation in vitro of M.26 apple rootstocks. J Hortic Sci 52:235–238
Jun J-H, Yae B-W, Yang M-H, Hwang J-H, Shin Y-U, Park J-B (1996) Influence of cultivar, light condition and pretreatment on adventitious shoot regeneration from leaves, internodes and petioles of Malus domestica Borkh. in vitro. J Korean Soc Hortic Sci 37:700–703
Kamboj JS, Blake PS, Quinlan JD, Webster AD, Baker DA (1997) Recent advances in studies on the dwarfing mechanism of apple rootstocks. Acta Hortic 451:75–82
Kamboj JS, Blake PS, Quinlan JD, Baker DA (1999) Identification and quantitation by GC-MS of zeatin and zeatin riboside in xylem sap from rootstock and scion of grafted apple trees. Plant Growth Regul 28:199–205
Keulemans J, de Witte K (1994) Plant regeneration from cotyledons and embryonic axes in apple: sites of reaction and effect of pre-culture in the light. In: Schimdt H, Kellerhals M (eds) Progress in temperate fruit breeding. Kluwer, Netherlands, pp 371–375
Kiss I, Jámbor-Benczúr E, Fehér T, Csillag F (1994) Anatomical characteristics of leaf surface and stomata of Hydrangea plants grown in vitro, in vivo and transplanted. Hortic Sci 26:98–100
Kiss I, Fehér T, Jámbor-Benczúr E (1997) Comparative anatomy of stems of Hydrangea macrophylla cv. Messalina in vitro and in vivo. Hortic Sci 29:74–78
Korban SS, Chen H (1992) Apple. In: Hammerschlag FA, Litz RE (eds) Biotechnology of perennial fruit crops. C.A.B. International, Wallingford, pp 203–227
Korban SS, O’Connor PA, Elobeidy A (1992) Effects of thidiazuron, naphthaleneacetic acid, dark incubation and genotype on shoot organogenesis from Malus leaves. J Hortic Sci 67:341–349
Kubalákova M, Strnad M (1992) The effects of aromatic cytokinins (populins) on micropropagation and regeneration of sugar beet in vitro. Biol Plant 34:578–579
Kulaeva ON, Burkhanova EA, Karavaiko NN, Selivankina SY, Porfirova SA, Maslova GG, Zemlyachenko YV, Börner T (2002) Chloroplasts affect the leaf response to cytokinin. J Plant Physiol 159:1309–1316
Lane WD (1978) Regeneration of apple plants from shoot meristem-tips. Plant Sci Lett 13:281–285
Li M, Leung DWM (2000) Starch accumulation is associated with adventitious root formation in hypocotyl cuttings of Pinus radiata. J Plant Growth Regul 19:423–428
Ling Q, Ming L, YongXi W, ZhenGuang H, GaiRong Z (2002) Effects of different cytokinins on leaves in vitro regeneration of Fuji 2001 apple variety. J Fruit Sci 19:215–218
Liu Q, Salih S, Hammerschlag F (1998) Etiolation of ‘Royal Gala’ apple (Malus x domestica Borkh.) shoots promotes high-frequency shoot organogenesis and enhanced β–glucuronidase expression from stem internodes. Plant Cell Rep 18:32–36
Magyar-Tábori K, Dobránszki J, Jámbor-Benczúr E, Bubán T, Lazányi J, Szalai J, Ferenczy A (2001) Post-effects of cytokinins and auxin levels of proliferation media on rooting ability of in vitro apple shoots (Malus domestica Borkh.) ‘Red Fuji’. Int J Hortic Sci 7:26–29
Mangat BS, Pelekis MK, Cassells AC (1990) Changes in the starch content during organogenesis in in vitro cultured Begonia rex stem explants. Physiol Plant 79:267–274
Masuda T, Bessho H, Komori S, Tsuchiya S (1988) Studies on cell culture and plant regeneration in apple. II. Adventitious shoot formation from the roots of intact micropropagated plantlets. Bulletin of the Fruit Research Station, Ministry of Agriculture, Forestry and Fisheries. Series C (Morioka). No. 15: 13–19
McAdam-O Connell D, Mac An tSaoir S, Copeland R (2004) Development of leaf disk regeneration system for ‘Bramley’s’ seedling apple (Malus x domestica Borkh.). Acta Hortic 663:483–486
McGaw BA, Burch LR (1995) Cytokinin biosynthesis and metabolism. In: Davies PJ (ed) Plant hormones, physiology, biochemistry and molecular biology. Kluwer, Dordrecht, pp 98–117
Modgil M, Handa R, Sharma DR, Thakur M (2005) High efficiency shoot regeneration from leaf explants of in vitro grown shoots of apple. Acta Hortic 696:123–128
Mok DW, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52:89–118
Montecelli S, Gentile A, Damiano C (2000) In vitro shoot regeneration of apple cultivar Gala. Acta Hortic 530:219–223
Ongjanov V (1988) Regeneracija biljaka jabuke kulturom lista in vitro. Regeneration of apple plants by in vitro leaf culture. Jugosl Vocar 22:423–426
Ordás RJ, Fernández B, Rodríguez R (1992) Benzyladenine-controlled protein synthesis and growth in apple cell suspensions. Physiol Plant 84:229–235
Ou CQ, Li LG, He P, Zhang ZH (2008) In vitro adventitious shoot regeneration and induction of tetraploid from leaves of Hanfu apple. J Fruit Sci 25:293–297
Pawlicki N, Welander M (1994) Adventitious shoot regeneration from leaf segments of in vitro cultured shoots of the apple rootstock Jork 9. J Hortic Sci 69:687–696
Pawlicki-Jullian N, Sedira M, Welander M (2002) The use of Agrobacterium rhizogenes transformed roots to obtain transgenic shoots of the apple rootstock Jork 9. Plant Cell Tissue Org Cult 70:163–171
Peng J, Peng F, Zhu C, Wei S (2008) Molecular cloning of a putative gene encoding isopentenyltransferase from pingyitiancha (Malus hupehensis) and characterization of its response to nitrate. Tree Physiol 28:899–904
Predieri S, Fasolo FFM (1989) High frequency shoot regeneration from leaves of the apple rootstock M 26 (Malus pumila Mill.). Plant Cell Tissue Org Cult 17:133–142
Qin L, Li M, Wang Y-X, Han L-X, Huang Z-G, Zhao G-R (2002) Effects of different cytokinins on leaves in vitro regeneration of Fuji 2001 apple variety. J Fruit Sci 19:215–218
Rugini E, Muganu M (1998) A novel strategy for the induction and maintenance of shoot regeneration from callus derived from established shoots of apple (Malus x Domestica Borkh.) cv. Golden Delicious. Plant Cell Rep 17:581–585
Saebo A, Krekling T, Appelgren M (1995) Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Org Cult 41:177–185
Saito A, Suzuki M (1999) Efficient shoot regeneration from calli of apple rootstock (Malus prunifolia var. ringo Asami Mo84-A) and cultivar (Malus x domestica cv. Fuji). J Plant Physiol 155:620–624
Sarwar M, Skirvin RM (1997) Effect of thidiazuron and 6-benzylaminopurine on adventitious shoot regeneration from leaves of three strains of ‘McIntosh’ apple (Malus X domestica Borkh.) in vitro. Sci Hortic 68:95–100
Sarwar M, Skirvin RM, Kushad M, Norton MA (1998) Selecting dwarf apple (Malus x domestica Borkh.) trees in vitro: multiple cytokinin tolerance expressed among three strains of ‘McIntosh’ that differ in their growth habit under field conditions. Plant Cell Tissue Org Cult 54:71–76
Schmidt G, Waldemaier S (1992) Histological studies on in vitro and ex vitro leaves during the adaptation phase of Rhododendron ‘Pink Pearl’. Acta Agron Hung 42:11–21
Sriskandarajah S, Goodwin P (1998) Conditioning promotes regeneration and transformation in apple leaf explants. Plant Cell Tissue Org Cult 53:1–11
Sriskandarajah S, Mullins MG (1981) Micropropagation of Granny Smith apple: factors affecting root formation in vitro. J Hortic Sci 56:71–76
Sriskandarajah S, Skirvin RM, Abu-Quaoud H, Korban SS (1990) Factors involved in shoot elongation and axillary shoots of three apple scion cultivars in vitro. J Hortic Sci 65:113–121
Sriskindarajah S, Goodwin PB, Speirs J (1994) Genetic transformation of the apple scion cultivar ‘Delicious’ via Agrobacterium tumefaciens. Plant Cell Tissue Org Cult 36:317–329
Staba EJ (1969) Plant tissue culture as a technique for the phytochemist. In: Seikel MK, Runeckles VC (eds) Recent advances in phytochemistry, vol 2. Appleton-Century Crofts, New York, pp 75–102
Standardi A, Houshmand D (1992) Effects of culture age and time of explant excision on in vitro shoot regeneration in an apple cultivar and apple rootstocks. Agric Mediterr 122:85–89
Strnad M, Hanuš J, Vaněk T, Kaminek M, Ballantine JA, Fussel B, Hanke DE (1997) Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus x Canadensis Moench., cv. Robusta). Phytocem 45:213–218
Sugiyama M (1999) Organogenesis in vitro. Curr Opin Plant Biol 2:61–64
Swartz HJ, Bors R, Mohamed F, Naess SK (1990) The effect of in vitro pretreatments on subsequent shoot organogenesis from excised Rubus and Malus leaves. Plant Cell Tissue Org Cult 21:179–184
Theiler-Hedtrich C, Theiler-Hedtrich R (1990) Influence of TDZ and BA on adventitious shoot regeneration from apple leaves. Acta Hortic 280:195–199
Van Staden J, Zazimalova E, George EF (2008) Plant growth regulators II: Cytokinins, their analogues and antagonists. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 205–226
Virscek-Marn M, Bohanec B, Javornik B (1999) Adventitious shoot regeneration from apple leaves: Optimisation of the protocol and assessment of genetic variation among regenerants. Phyton (Horn) 39:61–70
Watanabe M, Suzuki A, Komori S, Bessho H (2004) Comparison of endogenous IAA and cytokinins in shoots of columnar and normal type apple trees. J Japan Soc Hortic Sci 73:19–24
Webster CA, Jones OP (1991) Micropropagation of some cold-hardy dwarfing rootstocks for apple. J Hortic Sci 66:1–6
Welander M (1988) Plant regeneration from leaf and stem segments of shoots raised in vitro from mature apple trees. J Plant Physiol 132:738–744
Welander M, Maheswaran G (1992) Shoot regeneration from leaf explants of dwarfing apple rootstocks. J Plant Physiol 140:223–228
Werbrouck SPO, van der Jeugt B, Dewitte W, Prinsen E, Van Onckelen HA, Debergh PC (1995) The metabolism of benzyladenine in S. floribundum Schott ‘Petite’ in relation to acclimatisation problems. Plant Cell Rep 14:662–665
Werbrouck SPO, Strnad M, Van Onckelen HA, Debergh PC (1996) Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol Plant 98:291–297
Werner EM, Boe AA (1980) In vitro propagation of Malling 7 apple rootstock. Hortic Sci 15:509–510
Yancheva SD, Golubowicz S, Fisher E, Lev-Yadun S, Flaishman MA (2003) Auxin type and timing of application determine the activation of the developmental program during in vitro organogenesis in apple. Plant Sci 165:299–309
Yepes LM, Aldwinckle HS (1994) Factors that affect leaf regeneration efficiency in apple, and effect of antibiotics in morphogenesis. Plant Cell Tissue Org Cult 37:257–269
Ziv M, Chen J (2008) The anatomy and morphology of tissue cultured plants. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 465–477
Zobayed SMA, Armstrong J, Armstrong W (2001) Leaf anatomy of in vitro tobacco and cauliflower plantlets as affected by different types of ventilation. Plant Sci 161:537–548
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magyar-Tábori, K., Dobránszki, J., Teixeira da Silva, J.A. et al. The role of cytokinins in shoot organogenesis in apple. Plant Cell Tiss Organ Cult 101, 251–267 (2010). https://doi.org/10.1007/s11240-010-9696-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9696-6