Abstract
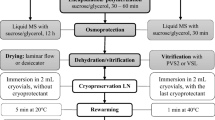
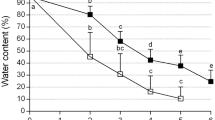
The first efficient cryopreservation procedure for in vitro-grown shoot tips of raspberry (Rubus idaeus L.) has been developed based on encapsulation–vitrification (EnVi) and encapsulation–dehydration (EnDe). EnVi resulted in higher survival (85%) and regrowth (75%) of cryopreserved shoot tips than EnDe (65 and 50%, respectively). In both cryogenic procedures, shoots regenerated from cryopreserved shoot tips without intermediary callus formation. Histological studies showed that a much larger number of meristematic cells survived following EnVi than EnDe. The EnVi procedure was applied to seven raspberry genotypes with an average survival and regrowth of 71 and 68%, respectively. Regenerated plants showed normal morphology. Results here indicate EnVi as a simple and efficient method for long-term preservation of R. idaeus germplasm.







Similar content being viewed by others
Abbreviations
- AC::
-
Activated charcoal
- BAP::
-
Benzylaminopurine
- BM::
-
Basic medium
- DMSO::
-
Dimethylsulphoxide
- IBA::
-
Indol-3-yl-acetic acid
- EnDe::
-
Encapsulation–dehydration
- EnVi::
-
Encapsulation–vitrification
- LN::
-
Liquid nitrogen
- MS::
-
Murashige and Skoog (1962) medium
- PVP::
-
Polyvinylpyrrolidone
- PVS2::
-
Plant vitrification solution 2
References
Chang Y, Reed BM (1999) Extended cold acclimation and recovery medium alteration improve regrowth of Rubus shoot tips following cryopreservation. Cryoletters 20:371–376
Crandall PC, Daubeny HA (1990) Raspberry management. In: Galletta GJ, Himelrick DG (eds) Small fruit crop management. Prentice Hall, Englewood Cliffs, N.J., pp 157–213
Crowe JH, Crowe LM, Carpenter JF, Wistrom CA (1987) Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J 242:1–10
Engelmann F (1997) In vitro conservation methods. In: Callow JA, Ford-Lloyd BV, Newbury HJ (eds) Biotechnology and plant genetic resources. CAB International, Oxford, pp 119–161
Fabre J, Dereuddre J (1990) Encapsulation–dehydration: a new approach to cryopreservation of Solanum shoot tips. Cryoletters 11:413–426
George EF, Sherrington PD (1984) Plant propagation by tissue culture. Exegetics, Eversley, UK
Gonzalez-Arnao MT, Engelmann F, Urra C, Morenza M, Rios A (1998) Cryopreservation of citrus apices using the encapsulation–dehydration technique. Cryoletters 19:177–182
Gurr E (1965) The rational use of dyes in biology and general staining methods. Hill, London
Hirai D, Sakai A (2003) Simplified cryopreservation of sweet potato [Ipomoea batatas (L.) Lam.] by optimizing conditions for osmoprotection. Plant Cell Rep 21:961–966
Hirai D, Shirai K, Shirai S, Sakai A (1998) Cryopreservation of in vitro-grown meristems of strawberry (Fragaria×ananassa Duch.) by encapsulation–vitrification. Euphytica 101:109–115
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (1997) Ultrastructural study on mechanism of increased freezing tolerance due to extracellular glucose in cabbage cells. Cryoletters 18:33–44
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (2002) Sucrose incubation increases freezing tolerance of asparagus (Asparagus officinalis L.) embryogenic cell suspensions. Cryoletters 23:103–112
Lambardi M, Fabbri A, Caccavale A (2000) Cryopreservation of white poplar (Populus alba L.) by vitrification of in vitro-grown shoot tips. Plant Cell Rep 19:213–218
Madhusudhanan K, Rahiman BA (2000) The effect of activated charcoal supplemented media to browning of in vitro cultures of Piper species. Biol Plant 43:297–299
Matsumoto T (2001) Cryopreservation of in vitro-cultured meristems of wasabi. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. JIRCAS, Japan, pp 212–216
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiol Plant 15:473–497
Niino T, Sakai A (1992) Cryopreservation of alginate-coated in vitro-grown shoot tips of apple, pear and mulberry. Plant Sci 87:199–206
Niino T, Tashiro K, Suzuki M, Ohuchi S, Magoshi J, Akihama T (1997) Cryopreservation of in vitro-grown shoot tips of cherry and sweet cherry by one-step vitrification. Sci Hortic 70:155–163
Paul H, Daigny G, Sangwan-Norreel BS (2000) Cryopreservation of apple (Malus×domestica Borkh.) shoot tips following encapsulation–dehydration or encapsulation–vitrification. Plant Cell Rep 19:768–774
Plessis P, Leddet C, Dereuddre J (1991) Resistance to dehydration and to freezing in liquid nitrogen of alginate coated shoot tips of grapevine (Vitis vinifera L. cv. Chardonnay). C R Acad Sci Paris Ser III 313:373–380
Poissonnier M, Monod V, Paques M, Dereuddre J (1992) Cryopreservation in liquid nitrogen of Eucalytus gunnii shoot tips grown in vitro following encapsulation and dehydration. Annales de Recherches Sylvicoles, AFOCEL, pp 5–23
Preil W, Engelhardt M (1979) Meristem culture of azaleas (Rhododendron simsii). Acta Hortic 78:203–207
Rall WF (1987) Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology 24:367–402
Reed BM (1988) Cold acclimation as a method to improve survival of cryopreserved Rubus meristems. Cryoletters 9:166–171
Reed BM (1993) Responses to ABA and cold acclimation are genotype dependent for cryopreserved blackberry and raspberry meristems. Cryobiology 30:179–184
Reed BM, Lagerstedt HB (1987) Freeze preservation of apical meristems of Rubus in liquid nitrogen. Hortic Sci 22:302–303
Sakai A, Nishiyama Y (1978) Cryopreservation of winter vegetative buds of hardy fruit trees in liquid nitrogen. Hortic Sci 13:225–227
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Tannoury M, Ralambosoa J, Kaminski M, Dereuddre J (1991) Cryopreservation by vitrification of coated shoot tips of carnation (Dianthus caryophyllus L.) cultured in vitro. C R Acad Sci Paris Ser III 313:633–638
Tapio E, Bremer K, Valkonen JPT (1997) Viruses and their significance in agricultural and horticultural crops in Finland. Agric Food Sci Finl 6:323–336
Vysotskaya ON, Mochammed AL, Butenko RG (1999) Cryopreservation of red raspberry meristems (Rubus idaeus L.) isolated from in vitro plantlets. Izvet Akad Nauk Ser Biol 1:25–29
Wang QC, Tang HR, Quan Y, Zhou GR (1994) Phenol induced browning and establishment of shoot tip explants of Fuji apple and Jinhua pear cultured in vitro. J Hortic Sci 69:833–839
Wang QC, Tanne E, Arav A, Gafny R (2000) Cryopreservation of in vitro-grown shoot tips of grapevine by encapsulation–dehydration. Plant Cell Tissue Org Cult 63:41–46
Wang QC, Batuman O, Li P, Bar-Joseph M, Gafny R (2002a) Cryopreservation of in vitro-grown shoot tips of ‘Troyer’ citrange [Poncirus trifoliata Raf.×Citrus sinensis (L.) Osbeck.] by encapsulation–dehydration. Plant Cell Rep 20:901–906
Wang QC, Batuman O, Li P, Bar-Joseph M, Gafny R (2002b) A simple and efficient cryopreservation of in vitro-grown shoot tips of ‘Troyer’ citrange [Poncirus trifoliata Raf.×Citrus sinensis (L.) Osbeck.] by encapsulation–vitrification. Euphytica 128:135–142
Wang QC, Mawassi M, Sahar N, Li P, Violeta C-T, Gafny R, Sela I, Tanne E, Perl A (2004) Cryopreservation of grapevine (Vitis spp.) embryogenic cell suspensions by encapsulation–vitrification. Plant Cell Tissue Org Cult 77:267–275
Acknowledgements
Financial support from the University of Helsinki and the Ministry of Agriculture and Forestry, Finland, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Lörz
Rights and permissions
About this article
Cite this article
Wang, Q., Laamanen, J., Uosukainen, M. et al. Cryopreservation of in vitro-grown shoot tips of raspberry (Rubus idaeus L.) by encapsulation–vitrification and encapsulation–dehydration. Plant Cell Rep 24, 280–288 (2005). https://doi.org/10.1007/s00299-005-0936-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0936-x