Abstract
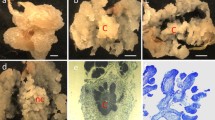
Agrobacterium-mediated genetic transformation has been widely used to generate transgenic plants in angiosperms. However, progress in conifer species has lagged because of the recalcitrant nature of gene transfer. In this study, a transgenic plant regeneration system has been established for slash pine (Pinus elliottii Engelm.) using Agrobacterium-mediated transformation. Among the different Agrobacterium tumefaciens strains (EHA105, GV3101, and LBA4404) tested, the highest frequency (60%) of transient β-glucuronidase-expressing embryos was obtained from Agrobacterium strain GV3101 with over 330 blue spots per embryo. To improve the frequency of transformation, different cocultivation conditions were analyzed. Combination of Agrobacterium density at OD600 = 0.9, 50 s sonication of embryos, and the addition of 50 μM acetosyringone produced the highest transformation efficiency, in which 56.2% of embryos formed hygromycin-resistant calli. Transient gene expression was observed in cotyledons and hypocotyls, but transgenic plants were only produced from callus cultures derived from embryonic cotyledons of transformed slash pine. Stable integration of transgenes in the plant genome of slash pine was confirmed by polymerase chain reaction, Southern blot, and Northern blot analyses. Transgenic lines with a single T-DNA copy were produced from Agrobacterium strains EHA105 (80.4%), GV3101 (95.7%), and LBA4404 (66%). These results demonstrated that a stable transformation system has been established in slash pine, and this system could provide an opportunity to transfer economically important genes into slash pine.







Similar content being viewed by others
References
Alves S. C.; Worland B.; Thole V.; Snape J. W.; Bevan M. W.; Vain P. A protocol for Agrobacterium-mediated transformation of Brachypodium distachyon community standard line Bd21. Nat. Protoc. 4: 638–649; 2009.
Birch R. G. Plant transformation: problems and strategies for practical application. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 297–326; 1997.
Boyko A.; Matsuoka A.; Kovalchuk I. High frequency Agrobacterium tumefaciens-mediated plant transformation induced by ammonium nitrate. Plant Cell Rep. 28: 737–757; 2009.
Cerda F.; Aquea F.; Gebauer M.; Medina C.; Arce-Johnson P. Stable transformation of Pinus radiata embryogenic tissue by Agrobacterium tumefaciens. Plant Cell Tiss. Organ Cult. 70: 251–257; 2002.
Cervera M.; Juarez J.; Navarro A.; Pina J. A.; Duran-Vila N.; Navarro L.; Pena L. Genetic transformation and regeneration of mature tissue of woody fruit plants bypassing the juvenile stage. Transgenic Res. 7: 51–59; 1998.
Charity J. A.; Holland L.; Donaldson S. S.; Grace L.; Walter C. Agrobacterium-mediated transformation of Pinus radiata organogenic tissue using vacuum-infiltration. Plant Cell Tiss. Organ Cult. 70: 51–60; 2002.
Cubitt A. B.; Heim R.; Adams S. R.; Boyd A. E.; Gross L. A.; Tsien R. Y. Understanding, improving, and using green fluorescent proteins. Trends Biochem. Sci. 20: 448–455; 1995.
Dillen W.; De Clercq J.; Kapila J.; Zambre M.; Van Montagu M.; Angenon G. The effect of temperature on Agrobacterium tumefaciens-mediated gene transfer to plants. Plant J. 12: 1459–1463; 1997.
Gelvin S. B. Agrobacterium in the genomics age. Plant Physiol. 150: 1665–1676; 2009.
Gould J. H.; Zhou Y. X.; Padmanabhan V.; Magallanes-Cedeno M. E.; Newton R. J. Transformation and regeneration of loblolly pine: shoot apex inoculation with Agrobacterium. Mol. Breed. 10: 131–141; 2002.
Gurel S.; Gurel E.; Kaur R.; Wong J.; Meng L.; Tan H. Q.; Lemaux P. G. Efficient, reproducible Agrobacterium-mediated transformation of sorghum using heat treatment of immature embryos. Plant Cell Rep. 28: 429–444; 2009.
Hajdukiewicz P.; Svab Z.; Maliga P. The small versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25: 989–994; 1994.
Halfhill M. D.; Richards H. A.; Mabon S. A.; Stewart Jr. C. N. Expression of uidA and Bt transgenes in Brassica napus and hybridization with Brassica rapa. Theor. Appl. Genet. 103: 659–667; 2001.
Hansen G.; Das A.; Chilton M.-D. Constitutive expression of the virulence genes improves the efficiency of plant transformation by Agrobacterium. Proc. Natl. Acad. Sci. U. S. A. 91: 7603–7607; 1994.
Huang Y.; Diner A. M.; Karnosky D. F. Agrobacterium rhizogenes-mediated genetic transformation and regeneration of a conifer: Larix decidua. In Vitro Cell Dev. Biol. Plant 27: 201–207; 1991.
James D. J.; Passey A. J.; Barbara D. J.; Bevan M. Genetic transformation of apple (Malus pumila Mill.) using a disarmed Ti-binary vector. Plant Cell Rep. 7: 658–661; 1989.
Jefferson R. A.; Kavanagh T. A.; Bevan M. W. GUS fusion: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907; 1987.
Klimaszewska K.; Lachance D.; Pelletier G.; Lelu M.-A.; Seguin A. Regeneration of transgenic Picea glauca, P. mariana, and P. abies after cocultivation of embryogenic tissue with Agrobacterium tumefaciens. In Vitro Cell Dev. Biol. Plant 37: 748–755; 2001.
Le V. Q.; Belles-Isles J.; Dusabenyagasani M.; Tremblay F. M. An improved procedure for production of white spruce (Picea glauca) transgenic plants using Agrobacterium tumefaciens. J. Exp. Bot. 52: 2089–2095; 2001.
Levee V.; Garin E.; Klimaszewska K.; Seguin A. Stable genetic transformation of white pine (Pinus strobus L.) after cocultivation of embryogenic tissues with Agrobacterium tumefaciens. Mol. Breed. 5: 429–440; 1999.
Maximova S. N.; Dandekar A. M.; Guiltinan M. J. Investigation of Agrobacterium-mediated transformation of apple using green fluorescent protein: High transient expression and low stable transformation suggest that factors other than T-DNA transfer are rate-limiting. Plant Mol. Biol. 37: 549–559; 1998.
Moore G. A.; Jacono C. C.; Neidigh J. L.; Lawrence S. D.; Cline K. Agrobacterium-mediated transformation of citrus stem segments and regeneration of transgenic plants. Plant Cell Rep. 11: 238–242; 1992.
Mourgues F.; Chevreau E.; Lambert C.; Bondt A. Efficient Agrobacterium-mediated transformation and recovery of transgenic plants from pear (Pyrus communis L.). Plant Cell Rep. 16: 245–249; 1996.
Peña L.; Cervera M.; Juárez J.; Navarro A.; Pina J. A.; Dura H. N.; Durán-Vila N.; Navarro L. Agrobacterium-mediated transformation of sweet orange and regeneration of transgenic plants. Plant Cell Rep. 14: 616–619; 1995.
Perl A.; Lotan O.; Abu-Abied M.; Holland D. Establishment of an Agrobacterium-mediated transformation system for grape (Vitis vinifera L.): the role of antioxidants during grape-Agrobacterium interactions. Nat. Biotechnol. 14: 624–628; 1996.
Sambrook J.; Fritsch E. F.; Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY; 1989.
Stachel S. E.; Messens E.; Montague M. V.; Zambryski P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318: 624–629; 1985.
Tang W.; Charles T. M.; Newton R. J. Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol. Biol. 59: 603–617; 2005.
Tang W.; Lin J.; Newton R. J. Okadaic acid and trifluoperazine enhance Agrobacterium-mediated transformation in eastern white pine. Plant Cell Rep. 26: 673–682; 2007.
Tang W.; Luo H.; Newton R. J. Effects of antibiotics on the elimination of Agrobacterium tumefaciens from loblolly pine (Pinus taeda) zygotic embryo explants and on transgenic plant regeneration. Plant Cell Tiss. Organ Cult. 70: 71–81; 2004.
Tang W.; Newton R. J.; Charles T. M. Plant regeneration through multiple adventitious shoot differentiation from callus cultures of slash pine (Pinus elliottii). J. Plant Physiol. 163: 98–101; 2006.
Trick H. N.; Finer J. J. Sonication-assisted Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill] embryogenic suspension culture tissue. Plant Cell Rep. 17: 482–488; 1998.
Walter C.; Grace L.; Donaldson S. S.; Moody J.; Gemmell J. E.; van der Maos S.; Kvaalen H.; Lonneborg A. An efficient biolistic transformation protocol for Picea abies embryogenic tissue and regeneration of transgenic plants. Can. J. For. Res. 29: 1539–1546; 1999.
Wenck A. R.; Quinn M.; Whetten R. W.; Pullman G.; Sederoff R. High-efficiency Agrobacterium-mediated transformation of Norway spruce (Picea abies) and loblolly pine (Pinus taeda). Plant Mol. Biol. 39: 407–416; 1999.
Zale J. M.; Agarwal S.; Loar S.; Steber C. M. Evidence for stable transformation of wheat by floral dip in Agrobacterium tumefaciens. Plant Cell Rep. 28: 903–913; 2009.
Acknowledgments
I would like to thank members of the Biotechnology Lab at Yangtze University for support. This work was supported by a grant from the education committee of Hubei Providence of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Tang, W., Xiao, B. & Fei, Y. Slash pine genetic transformation through embryo cocultivation with A. tumefaciens and transgenic plant regeneration. In Vitro Cell.Dev.Biol.-Plant 50, 199–209 (2014). https://doi.org/10.1007/s11627-013-9551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9551-7