Abstract
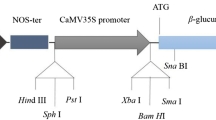
Three antibiotics were evaluated for their effects on the elimination of Agrobacterium tumefaciens during the genetic transformation of loblolly pine ( Pinus taeda L.) using mature zygotic embryos as targets. Agrobacterium tumefaciens strains, EHA105, GV3101, and LBA 4404, all harbouring the plasmid pCAMBIA1301, which carries the selectable marker gene, hygromycin phosphotransferase ( hpt) controlled by the cauliflower mosaic virus 35S promoter and terminator, and the uidA reporter gene (GUS) driven by the cauliflower mosaic virus 35S promoter and the terminator of nopaline synthase gene, were used in this study. Exposure to 350 mg l-1 carbenicillin, claforan, and timentin respectively for up to 6 weeks did not eliminate the Agrobacterium, while antibiotics at 500 mg l-1 eradicated them from the co-cultivated zygotic embryos. All three antibiotics increased callus growth and shoot regeneration at 350 and 500 mg l-1 each, but reduced callus growth and shoot regeneration at 650 mg l-1 when compared with controls. Putative transgenic calli were selected for continued proliferation and differentiation on 4.5 mg l-1 hygromycin-containing medium. Transformed calli and transgenic plants produced on a selection medium containing 4.5 mg l-1 hygromycin were confirmed by GUS histochemical assays, by polymerase chain reaction (PCR), and by Southern blot analysis. These results are useful for future studies on optimizing genetic transformation procedures in loblolly pine.
Similar content being viewed by others
References
Bais HP, George J & Ravishankar GA (1999) Influence of polyamines on growth of hairy root cultures of witloof chicory (Cichorium intybus L. cv. Lucknow Local) and formation of coumarins. J. Plant Growth Regul. 18: 33–37
Billings S, Jelenkovic G, Cjin CK & Eberhardt J (1997) The effect of growth regulators and antibiotics on eggplant transformation. J. Am. Soc. Hortic. Sci. 122: 158–162
Clapham DH & Ekberg I (1986) Induction of tumours by various strains of Agrobacterium tumefaciens on Abies nordmanniana and Picea abies. Scand. J. For Res. 1: 435–437
Dillen W, De Clercq J, Kapila J, Zambre M, Van Montagu M & Angenon G (1997) The effect of temperature on Agrobacterium tumefaciens-mediated gene transfer to plants. Plant J. 12: 1459–1463
Diner AM & Karnosky DF (1987) Differential responses of two conifers to in vitro inoculation with Agrobacterium rhizogenes. Eur. J. For Path. 17: 211–216
Ellis D, Roberts D, Sutton B, Lazaroff W, Webb D & Flinn B (1989) Transformation of white spruce and other conifer species by Agrobacterium tumefaciens. Plant Cell. Rep. 8: 16–20
Gupta PK, Dandekar AM & Durzan DJ (1988) Somatic proembryo formation and transient expression of a luciferase gene in Douglas fir and loblolly pine protoplasts. Plant Sci. 58: 85–92
Hansen G, Das A & Chilton M-D (1994) Constitutive expression of the virulence genes improves the efficiency of plant transformation by Agrobacterium. Proc. Natl. Acad. Sci. USA 91: 7603–7607
Hoekema A, Hirsch PR, Hooykaas PJJ & Schilperoort RA (1983) A binary plant vector strategy based on separation of vir and T-region of Agrobacterium tumefaciens Ti plasmid. Nature 303: 179–180
Holford P & Newbury HJ (1992) The effects of antibiotics and their breakdown products on the in vitro growth of Antirrhinum majus. Plant Cell. Rep. 11: 93–96
Hood EE, Gelvin SB, Melchers LS & Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 2: 208–218
Huang Y, Diner AM & Karnosky DF (1991) Agrobacterium rhizogenes-mediated genetic transformation and regeneration of a conifer: Larix deciduas. In Vitro Cell. Dev. Biol. 27: 201– 207
Humara JM, Lopez M & Ordas RJ (1999) Agrobacterium tumefaciens-mediated transformation of Pinus pinea L. cotyledons: An assessment of factors influencing the efficiency of uidA gene transfer. Plant Cell. Rep. 19: 51–58
Jefferson RA, Kavanagh TA & Bevan MW (1987) GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907
Klimaszewska K, Devantier Y, Lachance D, Lelu MA & Charest PJ (1997) Larix laricina (tamarack): somatic embryogenesis and genetic transformation. Can. J. For Res. 27: 538–550
Klimaszewska K, Lachance D, Pelletier G, Lelu M-A & Seguin A (2001) Regeneration of transgenic Picea glauca, P. mariana and P. abies after cocultivtion of embryogenic tissue with Agrobacterium tumefaciens. In Vitro Cell. Dev. Biol. Plant. 37: 748–755
Koncz C & Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204: 383–396
Le VQ, Belles-Isles J, Dusabenyagasani M & Tremblay FM (2001) An improved procedure for production of white spruce (Picea glauca) transgenic plants using Agrobacterium tumefaciens. J. Exp. Bot. 52: 2089–2095
Levee V, Garin E, Klimaszewska K & Seguin A (1999) Stable genetic transformation of white pine (Pinus strobus L.) after cocultivation of embryogenic tissues with Agrobacterium tumefaciens. Mol. Breed. 5: 429–440
Levee V, Lelu MA, Jouanin L, Cornu D & Pilate G (1997) Agrobacterium tumefaciens-mediated transformation of hybrid larch (Larix kaempferi × L. decidua) and transgenic plant regeneration. Plant Cell. Rep. 16: 680–685
Ling HQ, Kirseleit D & Ganal MW (1998) Effect of ticarcillin/ potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill). Plant Cell. Rep. 17: 843–847
McAfee BJ, White EE, Pelcher LE & Lapp MS (1993) Root induction in pine (Pinus) and larch (Larix) spp. using Agrobacterium rhizogenes. Plant Cell. Tiss. Org. Cult. 34: 53–62
Morris JW, Castle LA & Morris RO (1989) Efficacy of different Agrobacterium tumefaciens strains in transformation of pinaceous gymnosperms. Physiol. Mol. Plant Patho. 34: 451–461
Nauerby B, Billing K & Wyndaele R (1997) Influence of the antibiotic timentin on plant regeneration compared to carbenicillin and cefotaxime in concentration suitable for elimination of Agrobacterium tumefaciens. Plant Sci. 123: 169–177
Park SH, Lee BM, Salas MG, Srivatanakul M & Smith RH (2000) Shorter T-DNA or additional virulence genes improve Agrobactrium-mediated transformation. Theor. Appl. Genet. 101: 1015–1020
Rossi L, Horn B & Tinland B (1996) Integration of complete transferred DNA units is dependent on the activity of virulence E2 protein of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 93: 126–130
Sambrook J, Fritsch EF & Mamiatis T (1989) Molecular Cloning: A laboratory manual. 2nd ed., Cold Spring Harbor, NY
Sederoff R, Stomp AM, Chilton WS & Moore LW (1986) Gene transfer into loblolly pine by Agrobacterium tumefaciens. Biotech. 4: 647–649
Shin DI, Podila GK, Huang Y & Karnosky DF (1994) Transgenic larch expressing genes for herbicide and insect resistance. Can. J. For Res. 24: 2059–2067
Stomp AM, Loopstra C, Chilton WS, Sederoff RR & Moore LW (1990) Extended host range of Agrobacterium tumefaciens in the genus Pinus. Plant. Physiol. 92: 1226–1236
Teng WL & Nicholson L (1997) Pulse treatment of penicillin-G and streptomycin minimise internal infections and have posttreatment effects on the morphogenesis of ginseng root culture. Plant Cell. Rep. 16: 531–535
Tang W & Ouyang F (1999) Plant regeneration via organogenesis from six families of loblolly pine. Plant Cell. Tiss. Org. Cult. 58: 223–226
Tang H, Ren Z & Krczal G (2000). An evaluation of antibiotics for the elimination of Agrobacterium tumefaciens from walnut somatic embryos and for the effects on the proliferation of somatic embryos and regeneration of transgenic plants. Plant Cell. Rep. 19: 881–887
Tang W, Sederoff R & Whetten R (2001) Regeneration of transgenic loblolly pine (Pinus taeda L.) from zygotic embryos transformed with Agrobacterium tumefaciens. Planta. 213: 981–989
Tzfira T, Yarnitzky O, Vainstein A & Altman A (1996) Agrobacterium rhizogenes-mediated DNA transfer in Pinus halepensis Mill. Plant Cell. Rep. 16: 26–31
Wagner DB, Furnier GR, Saghai-Maroof MA, Williams SM, Dancik BW & Allard RW (1987) Chloroplast DNA polymorphisms in lodgepole and jack pine and their hybrids. Proc. Natl. Acad. Sci. USA 84: 2097–2100
Walter C, Grace L, Donaldson SS, Moody J, Gemmell JE, van der Maos S, Kvaalen H & Lonneborg A (1999) An efficient biolistic transformation protocol for Picea abies embryogenic tissue and regeneration of transgenic plants. Can. J. For Res. 29: 1539–1546
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tang, W., Luo, H. & Newton, R.J. Effects of antibiotics on the elimination of Agrobacterium tumefaciens from loblolly pine (Pinus taeda) zygotic embryo explants and on transgenic plant regeneration. Plant Cell, Tissue and Organ Culture 79, 71–81 (2004). https://doi.org/10.1007/s11240-004-4657-6
Issue Date:
DOI: https://doi.org/10.1007/s11240-004-4657-6