Abstract
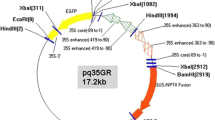
Mature zygotic embryos of recalcitrant Christmas tree species eastern white pine (Pinus strobus L.) were used as explants for Agrobacterium tumefaciens strain GV3101-mediated transformation using the uidA (β-Glucuronidase) gene as a reporter. Influence of the time of sonication and the concentrations of protein phosphatase inhibitor (okadaic acid) and kinase inhibitor (trifluoperazine) on Agrobacterium-mediated transformation have been evaluated. A high transformation frequency was obtained after embryos were sonicated for 45–50 s, or treated with 1.5–2.0 μM okadaic acid or treated with 100–200 μM trifluoperazine, respectively. Protein phosphatase and kinase inhibitors enhance Agrobacterium-mediated transformation in eastern white pine. A 2–3.5-fold higher rate of hygromycin-resistant callus was obtained with an addition of 2 μM okadaic acid or 150 μM trifluoperazine or sonicated embryos for 45 s. Stable integration of the uidA gene in the plant genome of eastern white pine was confirmed by polymerase chain reaction (PCR), Southern and northern blot analyses. These results demonstrated that a stable and enhanced transformation system has been established in eastern white pine and this system would provide an opportunity to transfer economically important genes into this Christmas tree species.





Similar content being viewed by others
Abbreviations
- BA:
-
N6-benzyladenine
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- hpt :
-
Hygromycin phosphotransferase gene
- uidA :
-
β-Glucuronidase
- IBA:
-
Indole-3-butyric acid
- PCR:
-
Polymerase chain reaction
References
Amoah BK, Wu H, Sparks C, Jones HD (2001) Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot 52:1135–1142
Birch RG (1997) Plant transformation: problems and strategies for practical application. Annu Rev Plant Physiol Plant Mol Biol 48:297–326
Cerda F, Aquea F, Gebauer M, Medina C, Arce-Johnson P (2002) Stable transformation of Pinus radiata embryogenic tissue by Agrobacterium tumefaciens. Plant Cell Tiss Org Cult 70:251–257
Cervera M, Juarez J, Navarro A, Pina JA, Duran-Vila N, Navarro L, Pena L (1998) Genetic transformation and regeneration of mature tissue of woody fruit plants bypassing the juvenile stage. Transgenic Res 7:51–59
Charity JA, Holland L, Donaldson SS, Grace L, Walter C (2002) Agrobacterium-mediated transformation of Pinus radiata organogenic tissue using vacuum-infiltration. Plant Cell Tiss Org Cult 70:51–60
Gould JH, Zhou YX, Padmanabhan V, Magallanes-Cedeno ME, Newton RJ (2002) Transformation and regeneration of loblolly pine: shoot apex inoculation with Agrobacterium. Mol Breeding 10:131–141
Hansen G, Wright MS (1999) Recent advances in the transformation of plants. Trends Plant Sci 4:226–231
Huang Y, Diner AM, Karnosky DF (1991) Agrobacterium rhizogenes-mediated genetic transformation and regeneration of a conifer: Larix decidua. In Vitro Cell Dev Biol 27:201–207
James DJ, Uratsu S, Cheng JS, Negri P, Viss P, Dandekar AM (1993) Acetosyringone and osmoprotectants like betaine or proline synergistically enhance Agrobacterium-mediated transformation of apple. Plant Cell Rep 12:559–563
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: b-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Klimaszewska K, Lachance D, Pelletier G, Lelu M-A, Seguin A (2001) Regeneration of transgenic Picea glauca, P. mariana and P. abies after cocultivation of embryogenic tissue with Agrobacterium tumefaciens. In Vitro Cell Dev Biol Plant 37:748–755
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10:165–174
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Levee V, Garin E, Klimaszewska K, Seguin A (1999) Stable genetic transformation of white pine (Pinus strobus L.) after cocultivation of embryogenic tissues with Agrobacterium tumefaciens. Mol Breeding 5:429–440
Le VQ, Belles-Isles J, Dusabenyagasani M, Tremblay FM (2001) An improved procedure for production of white spruce (Picea glauca) transgenic plants using Agrobacterium tumefaciens. J Exp Bot 52:2089–2095
Matthews PR, Wang MB, Waterhouse PM, Thornton S, Fieg SJ, Gubler F, Jacobsen JV (2001) Marker gene elimination from transgenic barley, using co-transformation with adjacent ‘twin T-DNAs’ on a standard Agrobacterium transformation vector. Mol Breeding 7:195–202
Miller M, Tagliani L, Wang N, Berka B, Bidney D, Zhao ZY (2002) High-efficiency transgene segregation in co-transformed maize plants using an Agrobacterium tumefaciens 2 T-DNA binary system. Transgenic Res 11:381–396
Moore GA, Jacono CC, Neidigh JL, Lawrence SD, Cline K (1992) Agrobacterium-mediated transformation of citrus stem segments and regeneration of transgenic plants. Plant Cell Rep 11:238–242
Mourgues F, Chevreau E, Lambert C, Bondt A (1996) Efficient Agrobacterium-mediated transformation and recovery of transgenic plants from pear (Pyrus communis L.). Plant Cell Rep 16:245–249
Noël GM, Tognetti JA, Pontis HG (2001). Protein kinase and phosphatase activities are involved in fructan synthesis initiation mediated by sugars. Planta 213:640–646
Pena L, Cervera M, Juarez J, Navarro A, Pina JA, Dura HN, Vila N, Navarro L (1995) Agrobacterium-mediated transformation of sweet orange and regeneration of transgenic plants. Plant Cell Rep 14:616–619
Pena L, Cervera M, Juarez J, Navarro A, Pina JA, Navarro L (1997) Genetic transformation of lime (Citrus aurantifolia Swing.): factors affecting transformation and regeneration. Plant Cell Rep 16:731–737
Perl A, Lotan O, Abu-Abied M, Holland D (1996) Establishment of an Agrobacterium-mediated transformation system for grape (Vitis vinifera L.): the role of antioxidants during grape–Agrobacterium interactions. Nat Biotechnol 14:624–628
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Plainview, NY
Shibata D, Liu YG (2000) Agrobacterium-mediated plant transformation with large DNA fragments. Trends Plant Sci 5:354–357
Tang W, Newton RJ (2005a) Transgenic Christmas trees regenerated from Agrobacterium tumefaciens-mediated transformation of zygotic embryos using the green fluorescence protein as a reporter. Mol Breeding 16:235–246
Tang W, Newton RJ (2005b) Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern eastern white pine (Pinus strobus L.) zygotic embryos. Plant Physiol Biochem 43:760–769
Tang W, Sederoff R, Whetten R (2001) Regeneration of transgenic loblolly pine (Pinus taeda L.) from zygotic embryos transformed with Agrobacterium tumefaciens. Planta 213:981–989
Tang W, Newton RJ, Lin J, Charles TM (2006) Expression of a transcription factor from Capsicum annuum in pine calli counteracts the inhibitory effects of salt stress on adventitious shoot formation. Mol Genet Genomics 276:242–253
Trick HN, Finer JJ (1997) SAAT: sonication-assisted Agrobacterium-mediated transformation. Transgenic Res 6:329–336
Wenck AR, Quinn M, Whetten RW, Pullman G, Sederoff R (1999) High-efficiency Agrobacterium-mediated transformation of Norway spruce (Picea abies) and loblolly pine (Pinus taeda). Plant Mol Biol 39:407–416
Wu H, Sparks C, Amoah B, Jones HD (2003) Factors influencing successful Agrobacterium-mediated genetic transformation of wheat. Plant Cell Rep 21:659–668
Acknowledgements
We would like to thank members of the Conifer Biotechnology Laboratory at East Carolina University for support and useful suggestions during this study. This work was supported by the Eastern North Carolina Christmas Tree Association.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. H. Wu
Rights and permissions
About this article
Cite this article
Tang, W., Lin, J. & Newton, R.J. Okadaic acid and trifluoperazine enhance Agrobacterium-mediated transformation in eastern white pine. Plant Cell Rep 26, 673–682 (2007). https://doi.org/10.1007/s00299-006-0270-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0270-y