Abstract
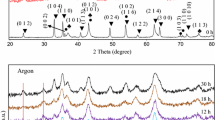
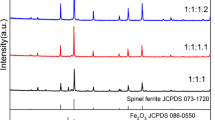
Spinel zinc ferrites ZnFe2O4, prepared by co-precipitation method using the zinc nitrate Zn(NO3)2·6H2O and ferric nitrate Fe(NO3)3·2H2O as the raw materials, were characterized by the thermo gravimetric analysis (TG) and differential scanning calorimeter (DSC), X-ray diffraction (XRD) and scanning electron microscope (SEM). The influence of synthesis conditions, such as Zn/Fe molar ratio, pH value, the sintering temperature and time, on the microstructures was detailedly investigated. The relationships between the microstructures and the synthesis conditions were discussed. The results show that the pure spinel zinc ferrites ZnFe2O4 are formed when the Zn/Fe molar ratio is 1.05:2 at pH=8.5 or Zn/Fe molar ratio is 1:2 at Ph=9-10, and the precursors are sintered at 1100 °C for 4 h. Especially no other phases are observed when the Zn/Fe molar ratio is 1:2 at pH=10 and the precursor is sintered above 700 for 4 °C h. The higher sintering temperature and longer sintering time contribute to grain growth.
Similar content being viewed by others
References
M Bohra, S Prasad, N Kumar, et al. Large Room Temperature Magnetization in Nanocrystalline Zinc Ferrite Thin Films[J]. Applied Physics Letters, 2006, 88: 62506
S Nakashima, K Fujita, K Tanaka, et al. First-principles XANES Simulations of Spinel Zinc Ferrite with a Disordered Cation Distribution[J]. Physical Review B (Condensed Matter), 2007, 75: 4443
J H Shim, S Lee, J H Park, et al. Coexistence of Ferrimagnetic and Antiferromagnetic Ordering in Fe-inverted Zinc Ferrite Investigated by NMR[J]. Physical Review B (Condensed Matter), 2006, 7306: 4404
S J Stewart, S J A Figueroa, J M Lopez, et al. Cationic Exchange in Nanosized ZnFe2O4 Spinel Revealed by Experimental and Simulated Near-edge Absorption Structure[J]. Physical Review B (Condensed Matter), 2007, 75: 3408
C Upadhyay, H C Verma. Anomalous Change in Electron Density at Nuclear Sites in Nanosize Zinc Ferrite[J]. Applied Physics Letters, 2004, 85: 2074–2076
J C Jung, H Lee, H Kim, et al. Oxidative Dehydrogenation of C-4 Raffinate-3 to 1,3-butadiene in a Dual-bed Reaction System Comprising ZnFe2O4 and Co9Fe3BiMo12O51 Catalysts: A Synergistic Effect of ZnFe2O4 and Co9Fe3BiMo12 O51 Catalysts[J]. Catalysis Letters, 2008, 123: 239–245
J Wolska, K Przepiera, H Grabowska, et al. ZnFe2O4 as a New Catalyst in the C-methylation of Phenol[J]. Research on Chemical Intermediates, 2008, 34: 43–51
F Braestrup, B C Hauback, K K Hansen. Temperature Dependence of the Cation Distribution in ZnFe2O4 Measured with High Temperature Neutron Diffraction[J]. Journal of Solid State Chemistry, 2008, 181: 2364–2369
J Gass, H Srikanth, N Kislov, et al. Magnetization and Magnetocaloric Effect in Ball-milled Zinc Ferrite Powder[J]. Journal of Applied Physics, 2008, 103: B7309
S H Yu, T Fujino, M Yoshimura. Hydrothermal Synthesis of ZnFe2O4 Ultrafine Particles with High Magnetization[J]. Journal of Magnetism and Magnetic Materials, 2003, 256: 420–442
M Jean, V Nachbaur. Determination of Milling Parameters to Obtain Mechanosynthesized ZnFe2O4[J]. Journal of Alloys and Compounds, 2008, 454: 432–436
F S Li, H B Wang, L Wang, et al. Magnetic Properties of ZnFe2O4 Nanoparticles Produced by a Low-temperature Solid-state Reaction Method[J]. Journal of Magnetism and Magnetic Materials, 2007, 309: 295–299
H Ehrhardt, S J Campbell, M Hofmann. Structural Evolution of Ball-milled ZnFe2O4[J]. Journal of Alloys and Compounds, 2002, 339: 255–260
H M Yang, X C Zhang, C H Huang, et al. Synthesis of ZnFe2O4 Nanocrystallites by Mechanochemical Reaction[J]. Journal of Physics and Chemistry of Solids, 2004, 65: 1329–1332
C N Chinnasamy, A Narayanasamy, N Ponpandian, et al. Magnetic Properties of Nanostructured Ferrimagnetic Zinc Ferrite[J]. Journal of Physics: Condensed Matter, 2000, 12: 7795–7805
J A Zhao, L W Mi, H W Hou, et al. The Preparation of Zinc Ferrite Nanorods by Using Single Ferrocenyl Complex as Precursor[J]. Materials Letters, 2007, 61: 4196–4198
X J Xu, L H Zhou, Q G Zhai, et al. Synthesis, Properties, and Formation Mechanism of Zinc Ferrite Hollow Spheres[J]. Journal of the American Ceramic Society, 2007, 90: 1959–1962
S Komarneni, M C D’Arrigo, C Leonelli, et al. Microwave-hydrothermal Synthesis of Nanophase Ferrites[J]. Journal of the American Ceramic Society, 1998, 81: 3041–3304
S H Zhan, C R Gong, D R Chen, et al. Preparation of ZnFe2O4 Nanofibers by Sol-gel Related Electrospinning Method[J]. Journal of Dispersion Science and Technology, 2006, 27: 931–933
G G Liu, X Z Zhang, Y J Xu, et al. Effect of ZnFe2O4 Doping on the Photocatalytic Activity of TiO2[J]. Chemosphere, 2004, 55: 1287–1291
S A Oliver, H H Hamdeh, J C Ho. Localized Spin Canting in Partially Inverted ZnFe2O4 Fine Powders[J]. Physical Review B (Condensed Matter), 1999, 60: 3400–3405
H Lee, J C Jung, H Kim, et al. Preparation of ZnFe2O4 Catalysts by a Co-precipitation Method Using Aqueous Buffer Solution and Their Catalytic Activity for Oxidative Dehydrogenation of n-butene to 1, 3-butadiene[J]. Catalysis Letters, 2008, 122: 281–286
M Maletin, E G Moshopoulou, A G Kontos, et al. Synthesis and Structural Characterization of In-doped ZnFe2O4 Nanoparticles[J]. Journal of the European Ceramic Society, 2007, 27: 4391–4394
J L M de Vidales, A Lopez-Delgado, E Vila, et al. The Effect of the Starting Solution on the Physico-chemical Properties of Zinc Ferrite Synthesized at Low Temperature[J]. Journal of Alloys and Compounds, 1999, 287: 276–283
S Ozcan, B Kaynar, M M Can, et al. Synthesis of ZnFe2O4 from Metallic Zinc and Iron by Wet-milling Process[J]. Materials Science and Engineering B-Solid State Materials for Advanced Technology, 2005, 121: 278–281
H Lee, J C Jung, H Kim, et al. Effect of pH in the Preparation of ZnFe2O4 for Oxidative Dehydrogenation of n-butene to 1, 3-butadiene: Correlation between Catalytic Performance and Surface Acidity of ZnFe2O4[J]. Catalysis Communications, 2008, 9: 1137–1142
J C Yu, J G Yu, W K Ho, Z T Jiang, L Z Zhang. Effects of F-Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater, 2002(14): 3808–3816
R Arulmurugan, G Vaidyanathan, S Sendhilnathan, B Jeyadevan. Mn-Zn Ferrite Nanoparticles for Ferrofluid Preparation: Study on Thermal-magnetic Properties[J]. Journal of Magnetism and Magnetic Materials, 2006(298): 83–94
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Science Foundation in China (No. 10804117) and Natural Science Foundation of Shanghai (No. 08ZR1421900)and the Major Program for the Fundamental Research of Shanghai (No. 06JC14033)
Rights and permissions
About this article
Cite this article
Ren, P., Zhang, J. & Deng, H. Preparation and microstructure of spinel zinc ferrite ZnFe2O4 by Co-precipitation method. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 24, 927–930 (2009). https://doi.org/10.1007/s11595-009-6927-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-009-6927-y