Abstract
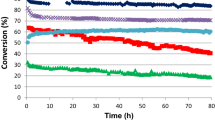
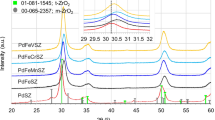
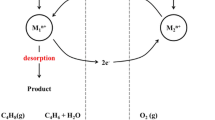
Zinc ferrite (ZnFe2O4) catalysts were prepared by a co-precipitation method using aqueous buffer solution with different pH (pH = 6−12), and applied to the oxidative dehydrogenation of n-butene to 1,3-butadiene. Conversion of n-butene and yield for 1,3-butadiene showed volcano-shaped curves with respect to pH value employed during the co-precipitation step. NH3-TPD experiments were conducted to correlate the acid property with the catalytic performance of zinc ferrite catalysts. It was revealed that the catalytic performance of zinc ferrite catalysts in the oxidative dehydrogenation of n-butene was closely related to the surface acidity of the catalysts. Conversion of n-butene and yield for 1,3-butadiene increased with increasing surface acidity of the catalysts. Among the catalysts tested, the zinc ferrite catalyst prepared at pH = 8 showed the best catalytic performance in the oxidative dehydrogenation of n-butene, which was attributed to its largest surface acidity.




Similar content being viewed by others
References
Oh SC, Lee HP, Kim HT, Yoo KO (1999) Korean J Chem Eng 16:543
Kung HH (1986) Ind Eng Chem Prod Res Dev 25:171
Hong F, Yang BL, Schwartz LH, Kung HH (1984) J Phys Chem 88:2525
Liaw BJ, Cheng DS, Yang BL (1989) J Catal 118:312
Jung JC, Lee H, Kim H, Chung Y-M, Kim TJ, Lee SJ, Oh S-H, Kim YS, Song IK (2007) J Mol Catal A 271:261
Van Oeffelen DAG, Van Hooff JHC, Schuit GCA (1985) J Catal 95:84
López Nieto JM, Concepción P, Dejoz A, Knözinger H, Melo F, Vázquez MI (2000) J Catal 189:147
Tiwari PN, Alkhazov TG, Adzamov KU, Khanmamedova AK (1989) J Catal 120:278
Rennard RJ, Kehl WL (1971) J Catal 21:282
Yang BL, Cheng DS, Lee SB (1991) Appl Catal 70:161
Toledo JA, Valenzuela MA, Armendáriz H, Aguilar-Ríos G, Zapata B, Montaya A, Nava N, Salas P, Schifter I (1995) Catal Lett 30:279
Toledo-Antonio JA, Nava N, Martínez M, Bokhimi X (2002) Appl Catal A 234:137
Kung HH, Kundalkar B, Kung MC, Cheng WH (1980) J Phys Chem 84:382
Kung HH, Kung MC (1985) Adv Catal 33:159
Jung JC, Lee H, Kim H, Chung Y-M, Kim TJ, Lee SJ, Oh S-H, Kim YS, Song IK (2007) Catal Commun. doi:10.1016/j.catcom.2007.07.031
Grasselli RK, Burrington JD (1981) Adv Catal 30:133
Cullis CF, Hucknall DJ (1982) In: Bond GC, Webb G (eds) A specialist periodical report: catalysis, vol 5. Royal Chemical Society, London, p 273
Massoth FE, Scarpiello DA (1971) J Catal 21:294
Lee H, Jung JC, Kim H, Chung Y-M, Kim TJ, Lee SJ, Oh S-H, Kim YS, Song IK (2007) Catal Commun. doi:10.1016/j.catcom.2007.10.023
Gibson MA, Hightower JW (1976) J Catal 41:420
Xu W-Q, Yin Y-G, Li G-Y, Chen S (1992) Appl Catal A 89:131
Ai M (1979) J Catal 60:306
Jeyadevan B, Chinnasamy CN, Shinoda K, Tohji K (2003) J Appl Phys 93:8450
Sharma RK, Suwalka O, Lakshmi N, Venugopalan K, Banerjee A, Joy PA (2005) Mater Lett 59:3402
Toledo-Antonio JA, Bosch P, Valenzuela MA, Montoya A, Nava N (1997) J Mol Catal A 125:53
Zhang M, Lan R, Liu J, Chen X, Zhou W (1992) J Chem Soc Faraday Trans 88:637
Acknowledgments
This work was supported by SK Energy Corporation (POST-BK21 Program) and Korea Energy Management Corporation (2005-01-0090-3-010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H., Jung, J.C., Kim, H. et al. Preparation of ZnFe2O4 Catalysts by a Co-precipitation Method Using Aqueous Buffer Solution and Their Catalytic Activity for Oxidative Dehydrogenation of n-Butene to 1,3-Butadiene. Catal Lett 122, 281–286 (2008). https://doi.org/10.1007/s10562-007-9371-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9371-7