Abstract
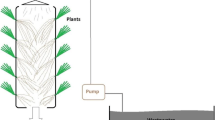
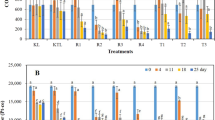
This research evaluated the feasibility of using vetiver plantlets (Vetiveria zizanioides (L.) Nash) on a floating platform with aeration to degrade phenol (500 mg/L) in illegally dumped industrial wastewater (IDIWW). The IDIWW sample was from the most infamous illegal dumping site at Nong Nae subdistrict, Phanom Sarakham district, Chachoengsao province, Thailand. Laboratory results suggested that phenol degradation by vetiver involves two phases: Phase I, phytopolymerization and phyto-oxidation assisted by root-produced peroxide (H2O2) and peroxidase (POD), followed by phase II, a combination of phase I with enhanced rhizomicrobial degradation. The first 360–400 h of phenol degradation were dominated by phytopolymerization and phyto-oxidation yielding particulate polyphenols (PPP) or particulate organic matter (POM) as by-products, while phenol decreased to around 145 mg/L. In Phase II, synergistically, rhizomicrobial growth was ∼100-folds greater on the roots of the vetiver plantlets than in the IDIWW and participated in the microbial degradation of phenol at this lower phenol concentration, increasing the phenol degradation rate by more than three folds. This combination of phytochemical and rhizomicrobiological processes eliminated phenol in IDIWW in less than 766 h (32 days), while without the vetiver plantlets, phenol degradation by aerated microbial degradation alone may require 235 days. To our knowledge, this is the first that systematically reveals the complete phenol degradation mechanism by vetiver plantlets in real aerated wastewater.





Similar content being viewed by others
References
Agostini E, Coniglio MS, Milrad SR, Tigier HA, Giulietti AM (2003) Phytoremediation of 2,4-dichlorophenol by Brassica napus hairy root cultures. Biotechnol Appl Biochem 37:139–144
Al-Khalid T, El-Naas M (2012) Aerobic biodegradation of phenols: a comprehensive review. Crit Rev Environ Sci Technol 42:1631–1690
Bahraminia M, Zarei M, Ronaghi A, Ghasemi-Fasaei R (2016) Effectiveness of arbuscular mycorrhizal fungi in phytoremediation of lead-contaminated soil by vetiver grass. Int J Phytoremediation 18(7):730–737
Bekins BA, Warren E, Godsy EM (1998) A comparison of zero-order, first-order, and Monod biotransformation models. Groundwater 36:261–268
Bootkote C (2013) Root of hazardous industrial waste problems; PCD does not have authority while DIW cannot identify the culprit, TCIJ. TCIJ, Bangkok
Brandt R, Merkl N, Schultze-Kraft R, Infante C, Broll G (2006) Potential of vetiver (Vetiveria zizanioides (L.) Nash) for phytoremediation of petroleum hydrocarbon-contaminated soils in Venezuela. Int J Phytoremediation 8:273–284
Chen Z, Cuervo DP, Müller JA, Wiessner A, Köser H, Vymazal J, Kästner M, Kuschk P (2016) Hydroponic root mats for wastewater treatment—a review. Environ Sci Pollut Res
Cook RL, Hesterberg D (2013) Comparison of trees and grasses for rhizoremediation of petroleum hydrocarbons. Int J Phytoremediation 15:844–860
Da Silva ML, Kamath R, Alvarez PJ (2006) Effect of simulated rhizodeposition on the relative abundance of polynuclear aromatic hydrocarbon catabolic genes in a contaminated soil. Environ Toxicol Chem 25:386–391
Danh LT, Truong P, Mammucari R, Tran T, Foster N (2009) Vetiver grass, Vetiveria zizanioides: a choice plant for phytoremediation of heavy metals and organic wastes. Int J Phytoremediation 11:664–691
Danh LT, Truong P, Mammucari R, Foster N (2010) Economic incentive for applying vetiver grass to remediate lead, copper and zinc contaminated soils. Int J Phytoremediation 13:47–60
De Araujo BS, Charlwood BV, Pletsch M (2002) Tolerance and metabolism of phenol and chloroderivatives by hairy root cultures of Daucus carota L. Environ Pollut 117:329–335
de Araujo BS, de Oliveira JO, Machado SS, Pletsch M (2004) Comparative studies of the peroxidases from hairy roots of Daucus carota, Ipomoea batatas, and Solanum aviculare. Plant Sci 167:1151–1157
Dudeja SS, Giri R, Saini R, Suneja-Madan P, Kothe E (2012) Interaction of endophytic microbes with legumes. J Basic Microb 52:248–260
EPA (2002) Toxicological review of phenol. US.EPA, Washington D.C.
Esplugas S, Gimenez J, Contreras S, Pascual E, Rodrı’guez M (2002) Comparison of different advanced oxidation processes for phenol degradation. Water Res 36:1034–1042
Garrity G, Boone DR, Castenholz RW (eds) (2001) Bergey’s manual of systematic bacteriology. Springer, New York, NY, p. 722
Ghioureliotis M, Nicell JA (2000) Toxicity of soluble products from the peroxidase-catalysed polymerization of substituted phenolic compounds. J Chem Technol Biotechnol 75:98–106
González PS, Maglione GA, Giordana M, Paisio CE, Talano MA, Agostini E (2012) Evaluation of phenol detoxification by Brassica napus hairy roots, using Allium cepa test. Environ Sci Pollut Res 19:482–491
González PS, Ontañon OM, Armendariz AL, Talano MA, Paisio CE, Agostini E (2013) Brassica napus hairy roots and rhizobacteria for phenolic compounds removal. Environ Sci Pollut Res 20:1310–1317
Gopalakrishnan G, Burken JG, Werth CJ (2009a) Lignin and lipid impact on sorption and diffusion of trichloroethylene in tree branches for determining contaminant fate during plant sampling and phytoremediation. Environ Sci Technol 43:5732–5738
Gopalakrishnan G, Werth CJ, Negri MC (2009b) Mass recovery methods for trichloroethylene in plant tissue. Environ Toxicol Chem 28:1185–1190
Harvey PJ, Campanella BF, Castro PM, Harms H, Lichtfouse E, Schäffner AR, Smrcek S, Werck-Reichhart D (2002) Phytoremediation of polyaromatic hydrocarbons, anilines and phenols. Environ Sci Pollut Res 9:29–47
Hill GA, Robinson CW (1975) Substrate inhibition kinetics: phenol degradation by pseudomonas putida. Biotechnol Bioeng 17:1599–1615
Huang JW, Chen J, Berti WR, Cunningham SD (1997) Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environ Sci Technol 31:800–805
Huang D-Y, Zhou S-G, Chen Q, Zhao B, Yuan Y, Zhuang L (2011) Enhanced anaerobic degradation of organic pollutants in a soil microbial fuel cell. Chem Eng J 172:647–653
Hussain A, Dubey SK, Kumar V (2015) Kinetic study for aerobic treatment of phenolic wastewater. Water Resour Indus 11:81–90
Ibáñez SG, Alderete LGS, Medina MI, Agostini E (2012) Phytoremediation of phenol using Vicia sativa L. plants and its antioxidative response. Environ Sci Pollut Res 19:1555–1562
Ichinose D, Yamamoto M (2011) On the relationship between the provision of waste management service and illegal dumping. Resour Energy Econ 33:79–93
Kamran MA, Syed JH, Eqani SA, Munis MF, Chaudhary HJ (2015) Effect of plant growth-promoting rhizobacteria inoculation on cadmium (Cd) uptake by Eruca sativa. Environ Sci Pollut Res 22:9275–9283
Kietkwanboot A, Tran THM, Suttinun O (2015) Simultaneous dephenolization and decolorization of treated palm oil mill effluent by oil palm fiber-immobilized Trametes Hirsuta strain AK 04. Water Air Soil Pollut 226:345
Klibanov AM, Tu T-M, Scott KP (1983) Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science 221:259–261
Kurnik K, Treder K, Skorupa-Kłaput M, Tretyn A, Tyburski J (2015) Removal of phenol from synthetic and industrial wastewater by potato pulp peroxidases. Water Air Soil Pollut 226:254
Kurzbaum E, Kirzhner F, Sela S, Zimmels Y, Armon R (2010a) Efficiency of phenol biodegradation by planktonic pseudomonas pseudoalcaligenes (a constructed wetland isolate) vs. root and gravel biofilm. Water Res 44:5021–5031
Kurzbaum E, Zimmels Y, Kirzhner F, Armon R (2010b) Removal of phenol in a constructed wetland system and the relative contribution of plant roots, microbial activity and porous bed. Water Sci Technol 62:1327–1334
Lee GF, Morris JC (1962) Kinetics of chlorination of phenol-chlorophenolic tastes and odors. Int J Air Wat Poll 6:419–431
Lei W, Xuanzhen J (2008) Unusual catalytic effects of iron salts on phenol degradation by glow discharge plasma in aqueous solution. J Hazard Mater 161:926–932
Massari M, Monzini P (2004) Dirty businesses in Italy: a case-study of illegal trafficking in hazardous waste. Global Crime 6:285–304
Moormann H, Kuschk P, Stottmeister U (2002) The effect of rhizodeposition from helophytes on bacterial degradation of phenolic compounds. Acta Biotechnol 22:107–112
Nielsen PH, Bjerg PL, Nielsen P, Smith P, Christensen TH (1995) In situ and laboratory determined first-order degradation rate constants of specific organic compounds in an aerobic aquifer. Environ Sci Technol 30:31–37
Oller ALW, Agostini E, Talano MA, Capozucca C, Milrad SR, Tigier HA, Medina MI (2005) Overexpression of a basic peroxidase in transgenic tomato (Lycopersicon esculentum mill. cv. Pera) hairy roots increases phytoremediation of phenol. Plant Sci 169:1102–1111
Ondo Zue Abaga N, Dousset S, Mbengue S, Munier-Lamy C (2014) Is vetiver grass of interest for the remediation of Cu and Cd to protect marketing gardens in Burkina Faso? Chemosphere 113:42–47
Poon K, Hon KL, Huang JJ (2011) The phytotoxicity of 2, 4, 6-Trichlorophenol and phenol to local agricultural plant species in China. WIT Trans Ecol Environ 152:203–213
Pradeep NV, Anupama S, Navya K, Shalini HN, Idris M, Hampannavar US (2015) Biological removal of phenol from wastewaters: a mini review. App Water Sci 5:105–112
Rentz JA, Alvarez PJ, Schnoor JL (2005) Benzo[a]pyrene co-metabolism in the presence of plant root extracts and exudates: implications for phytoremediation. Environ Pollut 136:477–484
Roongtanakiat N (2009) Vetiver phytoremediation for heavy metal decontamination. Office of the Royal Development Projects Board, Bangkok
Roongtanakiat N, Tangruangkiat S, Meesat R (2007) Utilization of vetiver grass (Vetiveria zizanioides) for removal of heavy metals from industrial wastewaters. Sci Asia 33:397–403
Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nature Biotechnol 13:468–474
Shibata A, Inoue Y, Katayama A (2006) Aerobic and anaerobic biodegradation of phenol derivatives in various paddy soils. Sci Total Environ 367:979–987
Singh S, Melo JS, Eapen S, D’Souza SF (2006) Phenol removal using Brassica juncea hairy roots: role of inherent peroxidase and H2O2. J Biotechnol 3:43–49
Singh S, Melo JS, Eapen S, D’Souza SF (2008) Potential of vetiver (Vetiveria zizanioides L. Nash) for phytoremediation of phenol. Ecotoxicol Environ Saf 71:671–676
Soonsuk D (2013) Diclosure of 40 toxic-waste contaminated area in the eastern Thailand, Thaipublica. Thaipublica, Bangkok
Stottmeister U, Wiessner A, Kuschk P, Kappelmeyer U, Kästner M, Bederski O, Müller RA, Moormann H (2003) Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol Adv 22:93–117
Talano MA, Busso DC, Paisio CE, González PS, Purro SA, Medina MI, Agostini E (2012) Phytoremediation of 2,4-dichlorophenol using wild type and transgenic tobacco plants. Environ Sci Pollut Res 19:2202–2211
Tong Z, Qingxiang Z, Hui H, Qin L, Yi Z, Min Q (1998) Kinetic study on the removal of toxic phenol and chlorophenol from waste water by horseradish peroxidase. Chemosphere 37:1571–1577
Triassi M, Alfano R, Illario M, Nardone A, Caporale O, Montuori P (2015) Environmental pollution from illegal waste disposal and health effects: a review on the “triangle of death”. Int J Environ Res Public Health 12:1216–1236
Truong PN, Baker D (1998) Vetiver grass system for environmental protection. Office of the Royal Development Projects Board, Bangkok
Truong P, Hart B (2001) Vetiver system for wastewater treatment, the PRVN secretariat office. Office of the Royal Development Projects Board (ORDPB), Bangkok
Truong P, Van TT, Pinners E (2007) Vetiver system applications: technical reference manual. The Vetiver Network International, San Antonio, Texas
Truong P, Van TT, Pinners E (2008) Vetiver grass-plant propagation, vetiver systems application—a technical reference manual. CreateSpace Independent Publishing Platform, Sydney, p. 100
US EPA (2010) Chapter 1, Part 141, National primary drinking water regulation. In: US.EPA (Hrsg.), Title 40-Protection of Environment, pp 341–565
Vaishnav DD, Babeu L, Korthals ET (1989) Effect of microbial concentration on biodegradation rates of phenols. J Ind Microbiol Biotechnol 4:307–313
Valipour A, Ahn Y-H (2016) Constructed wetlands as sustainable ecotechnologies in decentralization practices: a review. Environ Sci Pollut Res 23:180–197
van Schie PM, Young LY (2000) Biodegradation of phenol: mechanisms and applications. Biorem J 4:1–18
Veeramachaneni DN, Palmer JS, Amann RP (2001) Long-term effects on male reproduction of early exposure to common chemical contaminants in drinking water. Hum Reprod 16:979–987
Wright H, Nicell JA (1999) Characterization of soybean peroxidase for the treatment of aqueous phenols. Bioresour Technol 70:69–79
Zhang L, Zhao J, Cui N, Dai Y, Kong L, Wu J, Cheng S (2015) Enhancing the water purification efficiency of a floating treatment wetland using a biofilm carrier. Environ Sci Pollut Res 23(8):7437–7443
Acknowledgments
This research was funded by the Office of the Royal Development Projects Board through Naresuan University (Grant nos. R2558A092, R2557A057, and R2555B098). We are deeply thankful for excellent advice and continuous support from our expert consultants, including Dr. Weerachai Nanakorn, Ms. Suwanna Pasiri, and Dr. Pittayakorn Limthong. We deeply appreciate the trust from the Nong Nae community who welcomed our research team like a family. We appreciate Chachoengsao Development Station, Land Development Department, Ministry of Agriculture and Cooperatives, Thailand, for supporting vetiver grass for our research. Last but not least, we value the King of Thailand Vetiver Award (2015) and The Vetiver Network International (TVNI) Award (2015) granted to this research by the Chaipattana Foundation (Thailand) and the TVNI.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Gerald Thouand
Submitted to Environmental Science Pollution Research Special Issue on “Young Scholars in Earth and Environmental Sciences”
Electronic supplementary material
ESM 1
(DOCX 5270 kb)
Rights and permissions
About this article
Cite this article
Phenrat, T., Teeratitayangkul, P., Prasertsung, I. et al. Vetiver plantlets in aerated system degrade phenol in illegally dumped industrial wastewater by phytochemical and rhizomicrobial degradation. Environ Sci Pollut Res 24, 13235–13246 (2017). https://doi.org/10.1007/s11356-016-7707-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7707-9